Abstract
Introduction: FXI deficiency is characterised by a variable bleeding tendency. No clear correlation exists between bleeding and either FXI:C level or genotype. Some patients with major FXI deficiency (FXI:C <15 IU/dL) do not exhibit excessive bleeding, even after surgery or trauma, while others with partial deficiency (FXI:C 20-60 IU/dL) report significant haemorrhagic symptoms. The variation in bleeding tendency is observed between individuals with the same FXI:C level and also between individuals within families who share the same F11gene mutation. The thrombin generation (TG) assay quantifies the overall haemostatic potential of an individual through the measurement of thrombin production. While reduced TG has been observed in some FXI deficient individuals, there are conflicting reports about the ability of the TG assay to distinguish between patients with a history of bleeding (bleeders) and those without (non- bleeders).
Aims: To identify the optimal TG assay conditions which discriminate between normal controls and individuals with different levels of FXI deficiency. To investigate which conditions allow the TG assay to differentiate between bleeders and non-bleeders.
Methods: 97 adults with FXI deficiency were studied together with 50 controls. Bleeding histories were taken from each individual and citrated blood samples, both with addition of corn trypsin inhibitor (+ CTI) and without (- CTI). Platelet rich (PRP) and platelet poor plasmas (PPP) were prepared. TG was performed using the CAT method with tissue factor (TF) concentrations of 5pM, 1pM and 0.5pM in the following samples: PPP + CTI, PPP - CTI, PRP + CTI, PRP - CTI. TG results were compared between controls and 3 patient groups: partial FXI deficiency (FXI:C 20-60 IU/dL) (n=78), major low level FXI deficiency (FXI:C 3-15 IU/dL) (n=10) and major very low level FXI deficiency (FXI:C ≤2IU/dL) (n=9) using unpaired t-test or Mann Whitney U tests.
A subgroup of 74 patients was divided into bleeders and non-bleeders based solely on their experience of tonsillectomy and /or dental extraction prior to diagnosis of FXI deficiency. Those with excessive bleeding (requiring blood product transfusion or return to theatre/dentist for re-suturing or packing) were classified as bleeders (n=24) and those without, as non-bleeders (n=50). The optimal TG assay conditions to differentiate between bleeders and non-bleeders groups were identified with Receiver Operator Curves (ROC) using Area Under Curve (AUC) values.
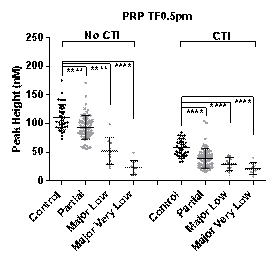
Results: TG performed in PPP with CTI was unable to differentiate between controls and all of the 3 patient groups. In all other sample types the best discrimination between controls and FXI deficient patients was seen at low TF (0.5pM) with a sequential reduction in peak height and ETP measurements observed as FXI:C levels decreased across the 3 patient groups (see Figure 1).
When TG results were compared between the bleeder (n=24) and non-bleeder (n=50) groups, peak height and ETP measured in PRP with CTI were best able to differentiate between the two groups (peak height ROC AUC = 0.9362, P<0.0001) (ETP ROC AUC=0.9362, P<0.0001). PPP samples and those without CTI were unable to segregate bleeders from non-bleeders as effectively.
Conclusions: In the presence of contact activation inhibition, FXI:C levels have minimal impact on thrombin generation in PPP but do influence thrombin generation in the presence of platelets. TG measured at low TF in PPP and PRP samples without CTI (with in vitro contact activation of FXI possible) was able to differentiate between controls and individuals with different levels of FXI deficiency but not between bleeders and non-bleeders.TG performed at low TF in PRP with CTI samples demonstrated the greatest differentiation between bleeders and non-bleeders in FXI deficiency providing further support for the clinical importance of platelet interaction with FXI in this disorder and suggesting that these test conditions may best mimic those in vivo.
Acknowledgments: This work is supported by a Fellowship Project Award (Bayer Hemophilia Awards Program), an unrestricted grant (LFB Biotechnologies) and a Wycherley Fellowship grant.
Pike:Bayer: Honoraria, Research Funding; LFB Biotechnologies: Honoraria, Research Funding. Bolton-Maggs:Bio-Products Laboratory (U.K.): Consultancy; LFB Biotechnologies: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.


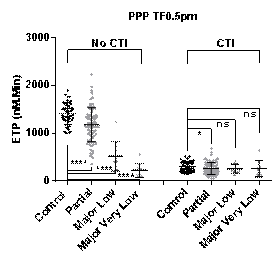
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal