Abstract
Background: ARS is characterized by body exposure to lethal amounts of penetrating radiation leading to stem cell depletion and other symptoms. Radiation that depletes bone marrow stem cells causes hematopoietic syndrome of ARS (HS-ARS), which leads to neutropenia, thrombocytopenia, and/or anemia and can impact OS because of increased rates of infection and/or hemorrhage. Filgrastim, a granulocyte colony-stimulating factor, decreases the duration and depth of absolute neutrophil count (ANC) suppression. Therefore, susceptibility to infection is reduced. In nonhuman primates (NHPs), filgrastim treatment resulted in significantly better survival rates (79% vs 40%) following irradiation (Farese AM, 2013). As human clinical trials are not feasible or ethical, we developed a model to project the impact of filgrastim treatment on human survival following acute radiation exposure based on virtual clinical trial simulation.
Methods: A mechanistic granulopoiesis model was developed to characterize granulopoietic homeostasis, the pharmacodynamic-mediated filgrastim disposition, and the impact of radiation and chemotherapy on ANCs in NHPs and humans. In NHPs, the granulopoiesis model quantified the ANC response to lethal amounts of radiation in filgrastim-treated (n=24) and untreated (n=22) animals (Farese AM, 2013). In humans, the same structural model was used to characterize the ANC response to filgrastim in healthy subjects and adult and pediatric patients (pts) with chemotherapy-induced neutropenia (CIN). A parametric time-to-event model relating ANC time course (± filgrastim) and OS hazard following irradiation in NHPs was established. To predict the expected survival benefit of filgrastim treatment in adults and pediatric pts with HS-ARS, the radiation component developed in NHPs was calibrated with historic mortality data for humans exposed to radiation (Scott BR, 1990). The radiation component was then used to replace the chemotherapy component of the human granulopoiesis model. The relative survival benefit (RSB), defined as the fraction of filgrastim-treated pts surviving relative to placebo 60 days after radiation exposure, was used to evaluate the projected efficacy of filgrastim treatment on OS in humans with HS-ARS in different scenarios using virtual clinical trial simulation.
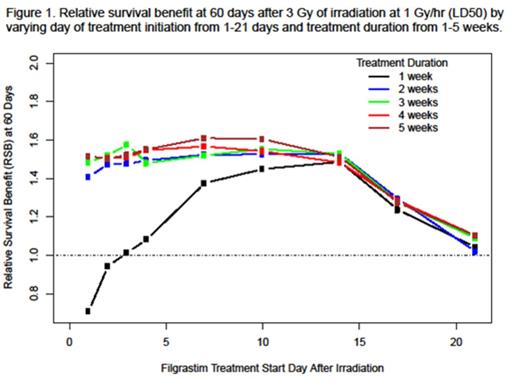
Results: The granulopoiesis model could characterize the ANC time course in irradiated NHPs ± filgrastim treatment and adequately describe human pharmacokinetic and ANC data from healthy subjects and pts with CIN. Similar model parameters between adults and pediatric pts were found with the exception of filgrastim volume of distribution and systemic clearance, which were scaled by body weight to adequately describe the ANC time course in pediatric pts. The OS model for irradiated NHPs was driven by the duration and depth of ANC suppression. The ANC time course accounted for 76% (95% CI: 41%, 97%) of the filgrastim treatment effect on OS. The scaled radiation injury and OS model developed for NHPs was capable of capturing human survival data over a wide range of radiation dose rates (0.01-1000 Gy/hr) in the absence of filgrastim treatment. Simulations predict that humans will be most at risk for neutropenia from day 10-21 after acute radiation exposure, and filgrastim treatment at the currently approved dose for CIN (subcutaneous [SC] 5 µg/kg once daily) administered for 2-3 consecutive weeks will increase the proportion of pts alive 60 days after acute radiation exposure by ~50% compared to placebo (Figure 1). Short durations of filgrastim treatment (< 2 weeks) initiated ≤ 3 days after acute radiation exposure or any duration of filgrastim treatment initiated > 14 days after acute radiation exposure will not provide maximal OS benefit. In addition, a duration of filgrastim treatment > 3 weeks, when initiated between day 4-14 after acute radiation exposure, will not provide additional OS benefit.
Conclusions: A single structural model was developed that described hematopoietic injury due to chemotherapy/radiation and the filgrastim treatment effect in NHPs and humans. Simulations of HS-ARS in adults and pediatric pts suggest that daily SC 5 µg/kg filgrastim treatment for 2-3 weeks will provide a relevant survival benefit compared to placebo if initiated within 14 days after acute radiation exposure. Increasing the daily filgrastim dose beyond 5 µg/kg/day will not provide additional OS benefit.
Harrold:Amgen Inc: Employment, Equity Ownership. Jacqmin:Amgen: Consultancy. Olsson:Amgen Inc.: Consultancy. Delor:Amgen Inc.: Consultancy. Morrow:Amgen Inc.: Employment, Equity Ownership. Yang:Amgen Inc.: Employment, Equity Ownership. Chow:Amgen Inc.: Employment, Equity Ownership. Perez-Ruixo:Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal