Abstract
BACKGROUND
Relapsed childhood B-ALL has a poor prognosis, with time to and site of relapse being the best clinical predictors of outcome. The role of allogeneic SCT is unclear for patients with late bone marrow (BM) or isolated extramedullary (IEM) relapse. We recently reported initial results from the AALL0433 trial for intermediate-risk relapse of childhood B-ALL, which established a 0.1% end-induction minimal residual disease (MRD) threshold as the best predictor of outcome (Lew, ASCO 2014). We now report an updated analysis, including outcomes for patients receiving SCT vs. continued chemotherapy.
METHODS
AALL0433 included patients with early CNS/testicular (IEM) relapse (<18 mo. from diagnosis), or late BM/combined relapse (≥36 mo. from diagnosis) of B-ALL, enrolling 271 eligible patients between 3/2007 and 10/2013. Therapy was based upon the earlier COG AALL01P2 / P9412 platforms. BM MRD was measured by flow cytometry at the end of Induction block 1, and for this analysis was considered positive (MRD+) if ≥0.1%, or negative (MRD-) if <0.1%. MRD testing was performed centrally by a COG reference lab, with results blinded to local investigators. 48 patients underwent matched family donor SCT per protocol after 3 induction blocks. An additional 31 patients were removed from protocol therapy to pursue off-study alternative donor SCT. Donor sources for these patients were 21 unrelated BM, 9 unrelated cord blood, and 1 haploidentical BM. The remaining 192 patients received chemotherapy (plus irradiation for those with EM involvement at relapse). Event free and overall survival (EFS/OS) comparisons for patients receiving chemotherapy vs. SCT were adjusted to start from median time to SCT (138 days) or the actual time of SCT if <138d. Patients who had events or dropped out before the adjusted starting time were excluded from the survival analyses.
RESULTS
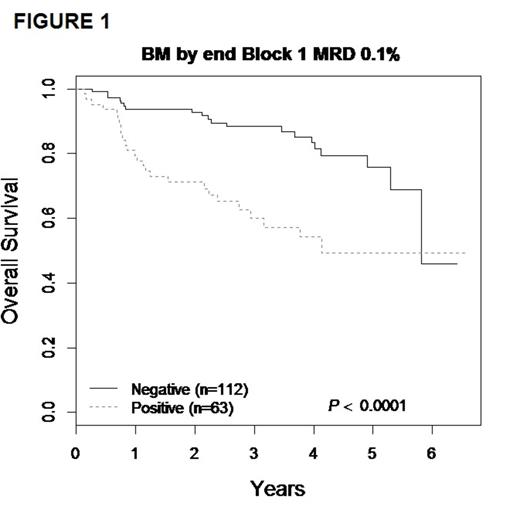
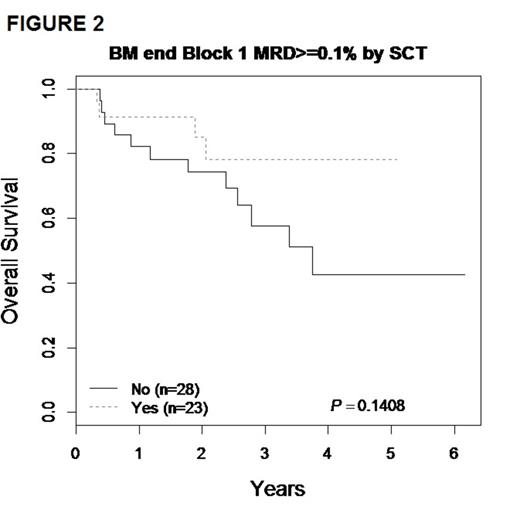
The 3-yr. EFS/OS for the entire cohort of 271 patients were 61.4 ± 4.3% and 72.9 ± 3.9% respectively. Focusing on patients with BM/combined relapse, the 3-yr EFS/OS for the 175 patients with available MRD data showed EFS/OS of 80.4 ± 4.7% and 88.3 ± 3.8% respectively for MRD- patients, compared to 45.1 ± 8.4% and 60.1% ± 8.3% for those who were MRD+ (p<0.01) (FIGURE 1). Because outcomes for patients who received matched family donor or alternative donor SCT were highly similar, these were pooled in all analyses of chemotherapy vs. SCT. There was no significant difference in survival for MRD- patients; 3-yr EFS and OS for the chemotherapy group were 75.4 ± 6.7% / 91.8 ± 4.3%, vs. 80.1 ± 8.9% / 84.0 ± 8.1% in the SCT group (p>0.5). In MRD+ patients the 3-yr EFS was 42.2 ± 13.1% vs 62.8 ± 13.5% (p=0.30) for the chemotherapy and SCT groups respectively, corresponding to a 3-yr OS of and 57.6 ± 12.5% vs 78.1 ± 12.9% (p=0.14), with a trend toward improved survival after 3 years with SCT (FIGURE 2).
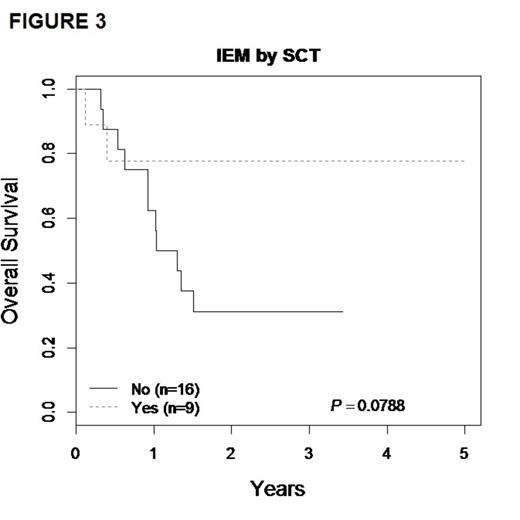
Although numbers were small, there was a clear trend toward improved outcomes in IEM patients receiving SCT over chemotherapy (FIGURE 3). 3-yr EFS for the chemotherapy vs. SCT groups were 31.3% ± 25.9% vs. 71.4% ± 22% (p=0.16), with OS of 31.3% ± 25.9% vs. 77.8% ± 21.2% (p=0.08). No IEM relapse patients were MRD+ at end induction.
CONCLUSIONS
Patients with late BM relapse of B-ALL who are MRD- after induction have a relatively good outcome (3-yr EFS of 80.4 ± 4.7%) on COG AALL0433. While additional follow-up is needed, there was no obvious benefit of SCT over chemotherapy for these patients. Outcomes were worse for BM relapse patients who remained MRD+ after induction (3-yr EFS 45.1 ± 8.4%). There was a trend toward benefit for SCT over chemotherapy for MRD+ patients. In the small number with early IEM, there was also a clear trend toward superiority of SCT over chemotherapy + radiotherapy. These data support the approach of reserving SCT for patients with late BM relapse of childhood B-ALL who remain MRD+, and also for patients with early IEM relapse.
Rheingold:Novartis: Consultancy. Whitlock:Glaxo-Smith-Kline: Research Funding. Borowitz:Becton Dickinson Biosciences: Research Funding. Hunger:Sigma Tau Pharmaceuticals: Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal