Abstract
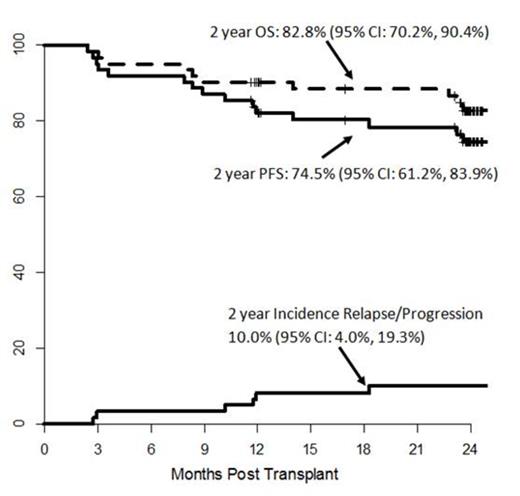
Allogeneic HCT has been shown to induce long term remissions in FL pts who have chemosensitive relapsed disease. The BMT CTN (sponsored by the NHLBI and NCI) conducted a multicenter phase 2 trial to examine the efficacy of rituximab (RTX)-containing RIC alloHCT (BMT CTN 0701: NCT 00912223). The primary objective was 2 year progression-free survival (PFS). Conditioning regimen consisted of fludarabine, cyclophosphamide and high-dose RTX (FCR) in which three* of the 4 doses of RTX were given IV at a dose of 1000 mg/m2 on days -13, -6*, + 1* and +8*. Graft vs host disease (GvHD) prophylaxis consisted of tacrolimus and mini-methotrexate. Sixty five patients who demonstrated chemosensitivity to the most recent salvage regimen were enrolled from 2009 to 2012 and 62 were evaluable. Median age was 55 years (range, 29-74). Matched related (47%) and unrelated (53%) donors provided mobilized blood allografts. This group was heavily pre-treated as 77% of pts received ≥ 3 prior regimens with 32% having received ≥ 5 prior regimens. No graft failures occurred and the overall response rate after HCT was 94% with 90% achieving a complete remission (CR), including 24 patients not in CR before alloHCT. With a median follow-up of 24 months (range, 12-31), the 2 year PFS and overall survival were 75% (95% confidence interval: 61%, 84%) and 83% (95% confidence interval: 70%, 90%) respectively. The 2 year cumulative incidence of relapse/progression and non-relapse mortality was 10% and 15%, respectively. The 2 year cumulative incidences of grade 2-4 and grade 3-4 acute GvHD were 27% and 10%, respectively, with a 55% incidence of extensive chronic GvHD. Of the 10 reported deaths, 1 was from relapse/progression, 6 were related to GvHD, 2 were related to infections and 1 unexpected death. Serum RTX concentrations were examined at 4 time points up to 1 year. Serum RTX levels peaked at day +28 and remained detectable as late as 1 year in 23 pts with available 1-year RTX level results (n=39). Blood absolute T lymphocytes including CD4 and CD8 subsets remained within normal range up to 1 year post-HCT; however, B-cell lymphocytes were significantly reduced or not detectable up to 1 year post-HCT. In conclusion, RIC allogeneic HCT with the FCR conditioning regimen confers high CR rates, a low incidence of relapse/progression and notable survival incidences in heavily pretreated FL patients. Serum RTX levels were detected in a majority of the patients for up to 1 yr post-HCT which may have contributed to the low incidence of relapse/progression.
Laport:Genentech: Research Funding. Off Label Use: fludarabine, cyclophosphamide, rituxiamb. Lazarus:Actinium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Celgene Pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal