Abstract
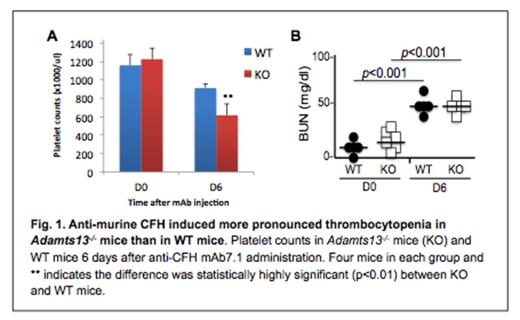
Thrombotic thrombocytopenic purpura (TTP), a potential fatal syndrome, is often associated with severe deficiency of plasma ADAMTS13 activity, either resulting from ADAMTS13 mutations or acquired anti-ADAMTS13 autoantibodies that inhibit plasma ADAMTS13 activity. Patients with severe ADAMTS13 do not always have TTP signs and symptoms, which often occur following infections or inflammatory responses. The mechanism of TTP flare is not fully understood. In the present study, complement activation markers (iC3b, C5b, Bb, and C4b) were determined by enzyme-linked absorbent assays (ELISA) in the initial plasmas (prior to plasma exchange) of 20 patients with acquired TTP with severe ADAMTS13 deficiency (less than 20% of normal) and plasmas from 20 healthy controls. Of 20 TTP patients, 19 exhibited positive inhibitor in the 50:50 mixing study. Plasma levels of iC3b (1,000 ± 1,062 ng/ml), sC5b-9 (1,342±867 ng/ml), and Bb (38.2±47.7 ng/ml), as well as C4b (74.3±49.5 ng/ml) in acquired TTP patients were significantly higher than those in healthy controls (p value less than 0.01) These results indicate that complement activation in both classic and alternative pathways is a common phenomenon in patients with acquired autoimmune TTP. To demonstrate the causative effect of complement activation in TTP, we turned to our Adamts13 null mice. C57BL/6 (Adamts13-/-) mice are resistant to the development of spontaneous and Shigatoxin-induced TTP syndrome. When injected with a murine specific monoclonal antibody against complement factor H (CFH) (800 micro grams/mouse), which inhibits binding of circulating CFH to endothelial cells and C3b, Adamts13-/- mice (C57BL/6) developed more severe thrombocytopenia and anemia than wild type mice did within 6 days without additional challenge. However, renal insufficiency manifested by the increase of plasma BUN concentration was similar in both groups (Fig. 1). These results indicate that complement activation through an alternative pathway, following antibody-mediated inhibition of CFH or other complement regulatory components, may trigger the onset of TTP in light of severe ADAMTS13 deficiency.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal