Abstract
Introduction: A systematic review of clinical trials investigating interventions used for the first-line treatment of patients with CLL who are not eligible for fludarabine-based therapy was undertaken and the feasibility of performing an adjusted indirect comparison (AIC) for OChl versus B, GA101Chl and RChl was explored.
Methods: Trials were identified by a systematic review; details of the methodology can be found in abstract B1333 of Hematologica (Woods et al, 2013). The trial designs, patient populations and treatment doses were examined to identify any evident systematic differences among the trials that might be expected to result in heterogeneity. The feasibility of performing an AIC using conventional methods and also alternative methods such as matching-adjusted indirect comparison (MAIC) was evaluated.
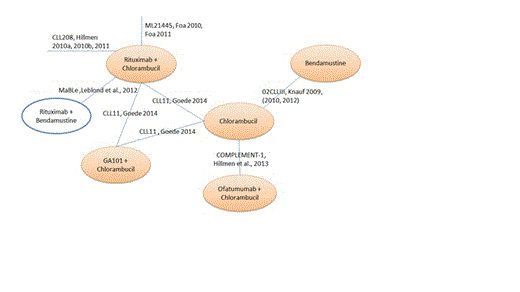
Results: The trial evidence network is presented in Figure 1.
The following trials were examined in detail to assess the feasibility of conducting an AIC with Chl as the common comparator.
µ COMPLEMENT-1 comparing OChl with Chl (Hillmen et al., 2013).
µ Knauf et al (2009; 2010, 2012) comparing B with Chl
µ CLL11 comparing RChl and GA101Chl with Chl (Goede et al., 2014).
Differences were found between studies in terms of treatment regimens, study design, subject characteristics and study endpoints. There were substantial differences in the actual median total dose of Chl monotherapy received by each patient, ranging from 728 mg in COMPLEMENT-1 through 522 mg for Knauf et al. to 384 mg in CLL11. Patient inclusion and exclusion criteria differed among trials, including disease stage (no Stage A patients in Knauf et al), absence or presence of criteria for fitness for fludarabine therapy, as determined by age or co-morbidity measurements such as the Cumulative Illness Rating Scale (CIRS) scores. The COMPLEMENT-1 population was older than that studied by Knauf et al. but younger than the population in the CLL11 trial. The majority of patients had ECOG Performance Status (PS) 1 in the COMPLEMENT-1 trial but PS 0 in the Knauf et al., trial. The definitions of response also varied among trials with COMPLEMENT-1 and CLL11 using the 2008 NCI-WG criteria and Knauf et al. using the earlier 1996 criteria.
Discussion: Naïve indirect comparisons are subject to a variety of confounding factors which may bias the estimates in an unpredictable direction with uncertain magnitude. There are a large number of differences between the identified trials which make adjustments for heterogeneity difficult. Different Chl monotherapy regimens have shown differing efficacy (Catovsky et al., 2011) and, there is no available evidence which might be used to calculate the magnitude of this effect. Methods such as MAIC and predictive models may overcome some of the heterogeneity between clinical trials and in some cases allow for comparisons of treatments in the absence of a common comparator. Within this set of studies, there was an insufficient set of matching variables to enable for matching and validation of the match with other variables and/or a common comparator arm. Furthermore, although these methods may account for differences in patient characteristics, they cannot adjust for other differences, such as the definition or ascertainment of endpoints.
Conclusions: To avoid bias, any indirect comparison of these interventions should adequately account for heterogeneity within the evidence network, with careful consideration of the factors identified in this review. The effectiveness of these interventions in comparison to Chl in routine clinical practice may differ from the efficacy observed in the trials, particularly where the Chl regimen used in routine practice differs from that investigated in the trials.
Wolowacz:GlaxoSmithKline. Sorrel Wolowacz is a full-time employee of RTI Health Solutions, which received funding from GlaxoSmithKline group of companies to conduct this study: Research Funding. Mitchell:GlaxoSmithKline. Sarah Mitchell is a full-time employee of RTI Health Solutions, which received funding from GlaxoSmithKline group of companies to conduct this study: Research Funding. Haiderali:GSK: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal