Abstract
Several studies have suggested a beneficial effect of CMV seropositivity and or reactivation on relapse risk after allogeneic hematopoietic cell transplantation (HCT) in myeloid malignancies. Inability to replicate this finding in T cell depleted HCT is suggestive of a potential effect modification between TCD and CMV in this setting, but this has not been addressed in previously published studies.
We have retrospectively analyzed transplant outcomes in 192 patients with myeloid malignancies who have undergone unrelated donor HCT at our center during January 2006 –November 2013. Among this 111 patients received in vivo TCD with low dose (30 mg) alemtuzumab and 81 patients received non-TCD transplants. Recipients were CMV seropositive in 57% of TCD transplants and 59% of non-TCD transplants.
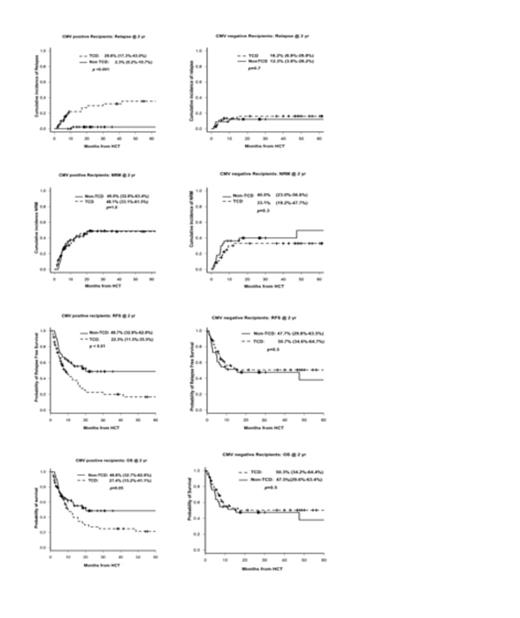
Analysis showed a significant effect modification (p=0.03 for interaction) between alemtuzumab and CMV serostatus on relapse risk, and stratified analysis based on recipient CMV status showed inferior outcomes with alemtuzumab in CMV seropositive recipients but not in CMV seronegative recipients. In CMV seropositive recipients alemtuzumab was an independent predictor of higher relapse (Hazard Ratio=13.95, p=0.008), inferior relapse free survival (HR=2.30, p=0.002), and inferior overall survival (HR= 2.04, p=0.008).
There was no difference in NRM between CMV seropositive and CMV seronegative groups, or between TCD and non-TCD transplants.
These findings suggest the presence of an effect modification by TCD on CMV's effect on relapse, resulting in inferior transplant outcomes with low dose Alemtuzumab in CMV seropositive recipients, but not in seronegative recipients.
CIR, NRM, RFS, and OS showing differential effects of T cell depletion between CMV seropositive and seronegative recipients
(A-B) Cumulative incidence of relapse, (C-D) Non-relapse mortality, (E-F) Relapse free survival, and (G-H) Over all survival.
CIR, NRM, RFS, and OS showing differential effects of T cell depletion between CMV seropositive and seronegative recipients
(A-B) Cumulative incidence of relapse, (C-D) Non-relapse mortality, (E-F) Relapse free survival, and (G-H) Over all survival.
Off Label Use: Alemtuzumab for GVHD prophylaxis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal