Abstract
Approximately 20% of Multiple Myeloma (MM) patients (pts) have renal dysfunction(RD) at the time of diagnosis and some more may develop it during the course of their disease. Autologous stem cell transplant (ASCT) is a treatment modality for this incurable malignancy and there are ongoing studies to determine the optimal timing of ASCT in this disease. There are conflicting data regarding the outcomes among different racial groups with regards to ASCT in MM. In this restrospective review we studied outcomes in pts of different races with RD undergoing ASCT for MM.
Between June 2005 and December 2013, we identified 107 pts with MM with RD (creatinine clearance < 60 ml/min/1.73 m2) who were not on hemodialysis and underwent ASCT at our institution. Of the 107 pts, 76 were caucasian(C) pts and 31 were identified as other (O) races (25 African American, one Hispanic, two Middle-eastern, three Asian). The patient characteristics of both groups are shown in Table 1. There were no statistically significant differences in the characteristics between the 2 groups. Approximately a quarter of the pts received their melphalan dose on the inpatient unit in both groups. During the hospitalization all pts received G-CSF from Day +6 till absolute neutrophil count ≥ 1500/µl and antimicrobial prophylaxis with norfloxacin, acyclovir and fluconazole.
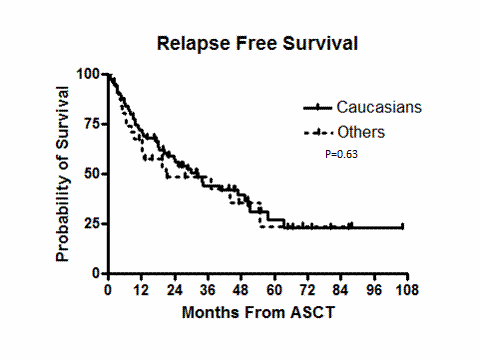
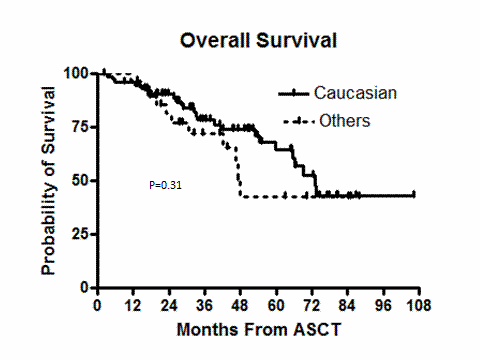
The median follow up was 35.9 months (range, 5.1-106.3). One patient in the C group died 98 days after ASCT and had evidence of disease progression. There were no deaths in the O group during the 100 days after ASCT. Table 2 shows post ASCT disease status and details about post ASCT maintenance therapy. There were no statistically significant differences between the groups in disease status or change in disease status at day 100 post ASCT. Although more patients in the C group received maintenance therapy post ASCT, this difference was not statistically significant. Figures 1 and 2 show the relapse free survival (RFS)and overall survival (OS) of both groups. The median RFS for C and O groups were 32.3 and 20.9 months (p =0.63, log rank), respectively. The median OS of the C and O groups were 73.1 and 47.8 month (p=0.31, log rank), respectively.
Our limited experience suggests that there was no effect of race in the post ASCT outcomes for MM pts with RD. ASCT was safe with acceptable transplant related mortality and good long -term outcomes for MM pts with renal dysfunction.
Patient Characteristics (N=107)
| Patient Characteristics . | Caucasians (n=76) . | Others (n=31) . | . | ||
|---|---|---|---|---|---|
| Median Age (range) | 63 (36-73) | 64(28-73) | 0.58 | ||
| Gender | Male | 42 (55%) | 17 (55%) | 0.9 | |
| Females | 35 (45%) | 14 (45%) | |||
| Chemo regimens prior to ASCT | 1 | 51 (67%) | 21 (68%) | 0.95 | |
| ≥ 2 | 25 (33%) | 10 (32%) | |||
| Agents used prior to ASCT | IMid | 12 (16%) | 4 (13%) | 0.85 | |
| PI | 18 (24%) | 9 (29%) | |||
| Both | 46 (60%) | 18 (58%) | |||
| Disease status at time of ASCT | PD | 7 (10%) | 5 (16%) | 0.35 | |
| SD | 9 (12%) | 4 (13%) | |||
| PR | 20 (26%) | 11 (35%) | |||
| VGPR | 20 (26%) | 8 (26%) | |||
| CR | 20 (26%) | 3 (10%) | |||
| Median creatinine clearance (range) | 45.5 (15-60) | 43 (21-60) | 0.35 | ||
| Subtype* | IgG Kappa | 29 (34%) | 18 (58%) | 0.25 | |
| IgG Lambda | 18 (24%) | 6 (19%) | |||
| Ig A Kappa | 9 (12%) | 2 (6%) | |||
| Ig A Lambda | 5 (7%) | 3 (10%) | |||
| K Light Chain | 6 (8%) | 0 | |||
| L Light Chain | 5 (7%) | 0 | |||
| Non- Secretory | 3 (4%) | 2 (6%) | |||
| Cytogenetic Risk | Standard | 65 (85%) | 18 (58%) | 0.67 | |
| High Risk | 5 (7%) | 2 (6%) | |||
| Median Melphalan Dose mg/m2 (range) | 140 (140-200) {140(58),160(5), 180(4),200(9)}® | 140 (100-200) {100(2),140(25), 200(9)} | 0.89 | ||
| Administration of Melphalan | Inpatient | 18 (24%) | 9 (29%) | 0.56 | |
| Outpatient | 58 (76%) | 22 (71%) | |||
| Median CD 34 Cell dose x106/kg (range) | 3.3 (2 -10.1) | 3.9 (2.3-9.5) | 0.34 | ||
| Median Engraftment Day (range) | Neutrophil | 12 (11-20) | 12(10-27) | 0.86 | |
| Platelets | 18(11-88) | 17 (11-42) | 0.10 | ||
| Median Days of Hospitalization (range) | 15 (9-65) | 16 (12-55) | 0.96 | ||
| Patient Characteristics . | Caucasians (n=76) . | Others (n=31) . | . | ||
|---|---|---|---|---|---|
| Median Age (range) | 63 (36-73) | 64(28-73) | 0.58 | ||
| Gender | Male | 42 (55%) | 17 (55%) | 0.9 | |
| Females | 35 (45%) | 14 (45%) | |||
| Chemo regimens prior to ASCT | 1 | 51 (67%) | 21 (68%) | 0.95 | |
| ≥ 2 | 25 (33%) | 10 (32%) | |||
| Agents used prior to ASCT | IMid | 12 (16%) | 4 (13%) | 0.85 | |
| PI | 18 (24%) | 9 (29%) | |||
| Both | 46 (60%) | 18 (58%) | |||
| Disease status at time of ASCT | PD | 7 (10%) | 5 (16%) | 0.35 | |
| SD | 9 (12%) | 4 (13%) | |||
| PR | 20 (26%) | 11 (35%) | |||
| VGPR | 20 (26%) | 8 (26%) | |||
| CR | 20 (26%) | 3 (10%) | |||
| Median creatinine clearance (range) | 45.5 (15-60) | 43 (21-60) | 0.35 | ||
| Subtype* | IgG Kappa | 29 (34%) | 18 (58%) | 0.25 | |
| IgG Lambda | 18 (24%) | 6 (19%) | |||
| Ig A Kappa | 9 (12%) | 2 (6%) | |||
| Ig A Lambda | 5 (7%) | 3 (10%) | |||
| K Light Chain | 6 (8%) | 0 | |||
| L Light Chain | 5 (7%) | 0 | |||
| Non- Secretory | 3 (4%) | 2 (6%) | |||
| Cytogenetic Risk | Standard | 65 (85%) | 18 (58%) | 0.67 | |
| High Risk | 5 (7%) | 2 (6%) | |||
| Median Melphalan Dose mg/m2 (range) | 140 (140-200) {140(58),160(5), 180(4),200(9)}® | 140 (100-200) {100(2),140(25), 200(9)} | 0.89 | ||
| Administration of Melphalan | Inpatient | 18 (24%) | 9 (29%) | 0.56 | |
| Outpatient | 58 (76%) | 22 (71%) | |||
| Median CD 34 Cell dose x106/kg (range) | 3.3 (2 -10.1) | 3.9 (2.3-9.5) | 0.34 | ||
| Median Engraftment Day (range) | Neutrophil | 12 (11-20) | 12(10-27) | 0.86 | |
| Platelets | 18(11-88) | 17 (11-42) | 0.10 | ||
| Median Days of Hospitalization (range) | 15 (9-65) | 16 (12-55) | 0.96 | ||
* 1 pt each in the caucasian group had IgD Lambda and IgM Kappa paraprotein
® Pts received 160 and 180 mg doses as part of a study
PI: proteosome inhibitors, PD: progressive disease, SD: stable disease, PR: partial response, VGPR: very good partial response, CR: Complete response
Disease status at Day 100 post ASCT
| . | Caucasian (N=76) . | Other (N=31) . | P Value . | |
|---|---|---|---|---|
| Disease Status | CR | 26 (34%) | 6 (19%) | 0.5 |
| VGPR | 20 (26%) | 12 (38%) | ||
| PR | 17 (22%) | 6 (19%) | ||
| SD | 7 (9%) | 4 (13%) | ||
| PD | 2 (3%) | 2 (7%) | ||
| Not available | 4 (6%) | 1 (3%) | ||
| Change in Disease Status | Improved | 23 (30%) | 13 (42%) | 0.66 |
| Unchanged | 45 (59%) | 15 (48%) | ||
| Worsened | 4 (6%) | 2 (7%) | ||
| Not available | 4 (6%) | 1 (3%) | ||
| Maintenance Therapy | IMid | 40 (53%) | 9 (29%) | 0.15 |
| PI | 2 (3%) | 1 (3%) | ||
| None | 29 (38%) | 19 (61%) | ||
| Not Known | 5 (7%) | 2 (7%) | ||
| . | Caucasian (N=76) . | Other (N=31) . | P Value . | |
|---|---|---|---|---|
| Disease Status | CR | 26 (34%) | 6 (19%) | 0.5 |
| VGPR | 20 (26%) | 12 (38%) | ||
| PR | 17 (22%) | 6 (19%) | ||
| SD | 7 (9%) | 4 (13%) | ||
| PD | 2 (3%) | 2 (7%) | ||
| Not available | 4 (6%) | 1 (3%) | ||
| Change in Disease Status | Improved | 23 (30%) | 13 (42%) | 0.66 |
| Unchanged | 45 (59%) | 15 (48%) | ||
| Worsened | 4 (6%) | 2 (7%) | ||
| Not available | 4 (6%) | 1 (3%) | ||
| Maintenance Therapy | IMid | 40 (53%) | 9 (29%) | 0.15 |
| PI | 2 (3%) | 1 (3%) | ||
| None | 29 (38%) | 19 (61%) | ||
| Not Known | 5 (7%) | 2 (7%) | ||
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal