Abstract
BACKGROUND: Peripheral T/NK–cell lymphomas (PTCL) are a heterogeneous group of neoplasms with a median survival of 2-3 years. Currently, there is not standard treatment although consolidation of response with autologous stem cell transplantation (ASCT) is recommended as front line therapy, except in anaplastic ALK+ and T/NK nasal localized lymphomas.
AIM: To analyze the outcome of patients with PTCL diagnosed and treated in our center between 2000 and 2012.
METHODS: An Intention to treat analysis has been performed to assess the response rate, progression-free survival (PFS) and overall survival (OS).
RESULTS: Thirty eight patients were identified in this period, 2 were excluded, 1 due to misdiagnosis and the other was not treated in our Department. There were 28 men and 8 women with a median age of 62 years (20-85 y), ECOG ≥ 2: 19%, B symptoms: 61%, IPI ≥ 2: 56% and PIT ≥2: 47%. The histological subtypes are described in Table 1.
Five patients died during diagnosis (aspergillosis, septic shock, intestinal perforation, Evans syndrome and hemophagocytic syndrome) and 2 rejected treatment. We treated 29 patients (80.5%) mainly with CHOP-like chemotherapy (77%) adding other schemas in order to achieve best response if needed.
In 15 patients ASCT was not indicated (9 due to age and 6 due to histological subtype). After induction, 4 patients achieved complete remission (CR), 3 partial response (PR) and 8 progressed (NR / P). Median number of treatments was 1 (range 1 to 3).
Fourteen patients were candidates for transplant but 4 of them could not receive it due to progression, early death, lost to follow-up and medical decision. Finally, 10 patients (71%) proceeded to transplant: 9 ASCT and 1 allo-SCT. The median number of treatments was 2 (1 - 3). Three patients were transplanted in CR, 6 PR and 1 NR/P; no patient improved the response. Three patients died during the procedure (RSV pneumonia, CMV and gram positive sepsis). The allo-SCT patient died of grade IV acute GVHD.
Currently, 7 out of 36 patients are alive in CR, 24 have died and in 5 were lost to follow-up. Causes of death were: lymphoma (58%), infection (25%), toxicity (4%) and unknown (13%). With a median follow-up for alive patients was 72 months, OS was 15.34 months (95% CI, 3.98-26.71) and PFS 13.61 months (95% CI, 10.15-17.07).
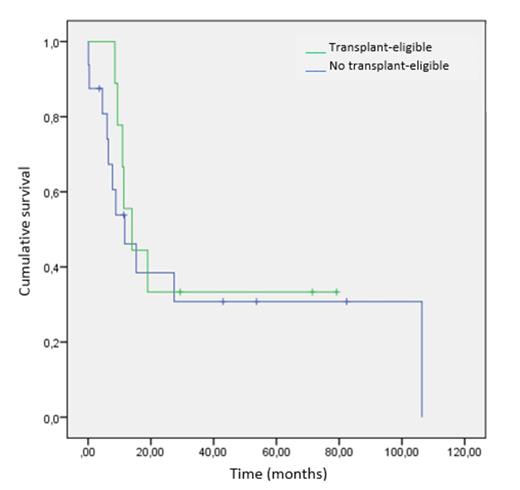
In Table 2 and figure 1 we show the median PFS and OS obtained in transplant- eligible and non-eligible patients.
CONCLUSIONS: We confirm the difficulty of long-term control of PTCL with current first-line treatments recommended by guidelines. Even in those patients receiving more intensive treatment with SCT, no improvement in PFS or OS was observed. New drugs and strategies are needed to improve the prognosis of these patients.
| HISTOLOGICAL SUBTYPES . | N= 36 . |
|---|---|
| Peripheral T-cell lymphoma NOS | 12 (33%) |
| Angioimmunoblastic T-cell lymphoma | 7 (19%) |
| Anaplastic large T-cell lymphoma, ALK+ | 6 (17%) |
| Anaplastic large T-cell lymphoma, ALK- | 5 (14%) |
| T/NK-cell lymphoma, nasal type | 4 (11%) |
| Intestinal T-cell lymphoma | 1 (3%) |
| Hepatosplenic γ/d T-cell lymphoma | 1 (3%) |
| HISTOLOGICAL SUBTYPES . | N= 36 . |
|---|---|
| Peripheral T-cell lymphoma NOS | 12 (33%) |
| Angioimmunoblastic T-cell lymphoma | 7 (19%) |
| Anaplastic large T-cell lymphoma, ALK+ | 6 (17%) |
| Anaplastic large T-cell lymphoma, ALK- | 5 (14%) |
| T/NK-cell lymphoma, nasal type | 4 (11%) |
| Intestinal T-cell lymphoma | 1 (3%) |
| Hepatosplenic γ/d T-cell lymphoma | 1 (3%) |
| . | SCT candidates . | No SCT candidates . |
|---|---|---|
| OS months (CI 95%) | 13.97 (6.40-21.53) | 11.67 (2.86-20.48) |
| PFS months (CI 95%) | 13.61 (7.09-20.12) | 11.51 (4.76-18.25) |
| . | SCT candidates . | No SCT candidates . |
|---|---|---|
| OS months (CI 95%) | 13.97 (6.40-21.53) | 11.67 (2.86-20.48) |
| PFS months (CI 95%) | 13.61 (7.09-20.12) | 11.51 (4.76-18.25) |
Alegre:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jansen: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal