Abstract
Second autologous stem cell transplant (ASCT) is a treatment option for multiple myeloma (MM) patients (pts) who have disease progression after first ASCT. About 20% of MM pts have evidence of renal dysfunction and many more develop it subsequently. Outcomes of second ASCT in pts with renal dysfunction have not been described.
We identified 18 pts at our institution who underwent a second ASCT for relapsed MM and had evidence of renal dysfunction (creatinine clearance ≤ 60ml/min/1.73m2). Pts who underwent a planned tandem transplant were excluded from this analysis. Patient characteristics are shown in Table 1. The median age of pts was 61 years (range, 49-72). The median time between first and second ASCT was 49.8 months (range, 19.2-81.9). Ten pts received one chemotherapy regimen while nine pts received two or more chemotherapy regimens between first and second ASCT. The chemotherapy regimens used between first and second transplant included IMid based (n=6), proteosome inhibitor based (n=3) and combination of both (n=9). Approximately half of the pts had progressive disease at the time of second ASCT. Median creatinine clearance at the time of second ASCT was 43.5 ml/min//1.73m2. All pts received G-CSF 5µg/Kg from day + 6 till absolute neutrophil count was ≥1500/µL, norfloxacin, acyclovir and fluconazole were used for antimicrobial prophylaxis during the hospitalization. One pt was dialysis dependent. Patients received a median dose of melphalan of 140mg/m2 (range 100-200 mg/m2).
Median follow up post second ASCT was 17.1 months (range, 2.4- 100). One pt died 74 days after the transplant due to multi- organ failure. Disease status at day 100 post ASCT is shown in Table 2. Thirteen of 17 pts had a partial response or better after second ASCT. Of 17 evaluable pts at day 100, twelve had an upgrade from the disease response category achieved with the last regimen used prior to second ASCT while five pts had stable disease response. Three pts received maintenance therapy after the second ASCT with bortezomib(n=2) and lenalidomide (n=1). Figures 1 and 2 show the overall survival (OS) and relapse free survival (RFS). Median RFS was 22.2 months and median OS was 27 months.
The outcomes of patients with renal dysfunction who underwent ASCT in the era of novel agents at our institution are comparable to those reported for second ASCT. The transplant related mortality was not higher than expected and the median PFS of about 2 years makes the option of second ASCT in this group of patients a valuable treatment modality. The utility of maintenance therapy after the second ASCT is not established, but may have contributed to the durable responses seen in some pts assessed herein and deserves to be investigated further.
Pt Characteristics (N=18)
| Patient Characteristics . | N (%) . | |
|---|---|---|
| Median Age (range) | 61 years(49-72) | |
| Median Time from First ASCT (range) | 49.8 months(19.2-81.9) | |
| Gender | Male | 9(50%) |
| Females | 9 (50%) | |
| Race | Caucasians | 13 (72%) |
| Others | 5 (28%) | |
| Disease status at time of ASCT | PD | 9 (50%) |
| SD | 5 (28%) | |
| PR | 1 (5.5 %) | |
| VGPR | 2 (11%) | |
| CR | 1 (5.5%) | |
| Median creatinine clearance (range) | 43.5 (5-59) | |
| Subtype | IgG Kappa | 10 (56%) |
| IgG Lambda | 6 (33%) | |
| Kappa Light Chain | 2 (11%) | |
| Cytogenetic Risk | Standard | 10 (56%) |
| High Risk | 4(22%) | |
| Unknown | 4 (22%) | |
| Melphalan Dose median mg/m2( range) | 140 (100-200) {100(2pts), 140(10pts), 180(1pt), 200(4pts)} | |
| Median CD 34 Cell dose x106/kg (range) | 4.66(2.6-27.5) | |
| Median Engraftment Day (range) | WBC | 11 (9-16) |
| Platelets | 21 (12-61) | |
| Median Days of Hospitalization (range) | 19(11-74) | |
| Patient Characteristics . | N (%) . | |
|---|---|---|
| Median Age (range) | 61 years(49-72) | |
| Median Time from First ASCT (range) | 49.8 months(19.2-81.9) | |
| Gender | Male | 9(50%) |
| Females | 9 (50%) | |
| Race | Caucasians | 13 (72%) |
| Others | 5 (28%) | |
| Disease status at time of ASCT | PD | 9 (50%) |
| SD | 5 (28%) | |
| PR | 1 (5.5 %) | |
| VGPR | 2 (11%) | |
| CR | 1 (5.5%) | |
| Median creatinine clearance (range) | 43.5 (5-59) | |
| Subtype | IgG Kappa | 10 (56%) |
| IgG Lambda | 6 (33%) | |
| Kappa Light Chain | 2 (11%) | |
| Cytogenetic Risk | Standard | 10 (56%) |
| High Risk | 4(22%) | |
| Unknown | 4 (22%) | |
| Melphalan Dose median mg/m2( range) | 140 (100-200) {100(2pts), 140(10pts), 180(1pt), 200(4pts)} | |
| Median CD 34 Cell dose x106/kg (range) | 4.66(2.6-27.5) | |
| Median Engraftment Day (range) | WBC | 11 (9-16) |
| Platelets | 21 (12-61) | |
| Median Days of Hospitalization (range) | 19(11-74) | |
Disease status at Day 100 post ASCT
| Disease status at Day 100 post ASCT | N(%) | |
| CR | 4 (22%) | |
| VGPR | 4 (22%) | |
| PR | 5 (28%) | |
| SD | 3 (17%) | |
| PD | 1 (5.5%) | |
| NOT AVAILABLE * | 1 (5.5%) |
| Disease status at Day 100 post ASCT | N(%) | |
| CR | 4 (22%) | |
| VGPR | 4 (22%) | |
| PR | 5 (28%) | |
| SD | 3 (17%) | |
| PD | 1 (5.5%) | |
| NOT AVAILABLE * | 1 (5.5%) |
PD: Progressive disease, SD: Stable disease; PR: Partial response; VGPR: Very good partial response; CR: complete response; *Pt died at day 74 post second ASCT
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

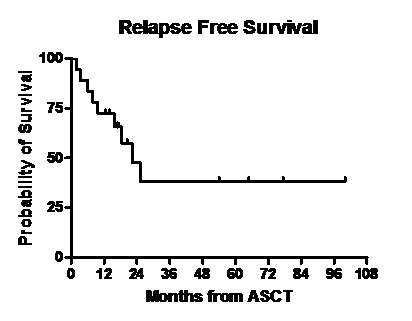
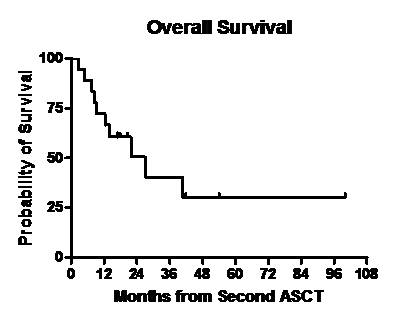
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal