Abstract
Background: The female donor/male recipient combination increases the risks of graft-versus-host disease (GVHD) and non-relapse mortality (NRM) after allogeneic stem cell transplantation (allo-SCT), with a possible deleterious impact on overall survival (OS). The mammalian Y chromosome encodes male-specific minor histocompatibility (H-Y) antigens that are recognized by female T cells. Some clinical data suggest a strong correlation between H-Y Antigen disparity and GVHD. In addition, female donors are notorious for bearing memory type lymphocytes induced by previous pregnancies; such allo-immune cells may provoke unwanted immune reactions such as GVHD in the recipient. To explore the impact of Female related donor (F-MRD) versus male matched unrelated donor (M-MUD) on outcome of peripheral blood allo-SCT in male recipients, we undertook a single center retrospective analysis of male adult patients transplanted at our center in the last 10 years. MUDs were matched at the allele level for HLA-A, B, C, DRB1, and DQB1. We excluded patients who received donor lymphocyte infusion post SCT.
Methods: Disease-free survival (DFS) and OS were calculated using the Kaplan-Meier estimate. Cumulative incidences (CI) were used for relapse (REL), and GVHD in a competing risks setting, NRM being a competing event for REL, and death for GVHD. The following variables were considered: recipient age (³ vs < 57 years, median age), F-MRD vs M-MUD, myeloablative (MAC) vs reduced-intensity ablative conditioning (Red ABL) vs non-myeloablative (NMA) regimen, early (CR1 or PR1 or chronic phase or untreated) vs advanced disease. Steroid-refractory (SR) GVHD was defined as acute GVHD progressing after 3 days of treatment, or unchanged after 7 days, or in incomplete response after 14 days, or chronic GVHD with no response after one month, no partial response after 2 months or progressive disease in 2 weeks of initiation of steroid treatment or during the methylprednisolone taper.
RESULTS: Fifty-six patients were identified and included in this analysis. The median age at transplant was 57 years (19-73). Diseases were AML (n=21), ALL (n=7), NHL (n=9), Hodgkin's disease (n=1), myeloma (n=5), MDS (n=11), CLL (n=1), and CML (n=1). Conditioning regimens were MAC (n=35), RED ABL (n=17) or NMA (n=4). Donors were F- MRD (n=24) or M-MUD (n=32). Source of stem cells was peripheral blood in all patients. GVHD prophylaxis consisted of tacrolimus and methotrexate in MRD and tacrolimus, methotrexate and anti-thymocyte globulin in MUD. Of the female donors, 63% had previous pregnancies before donation, 8% had no pregnancies and 29% were unknown. Mean age of female donors was 48 while the mean age for male donors was 35.
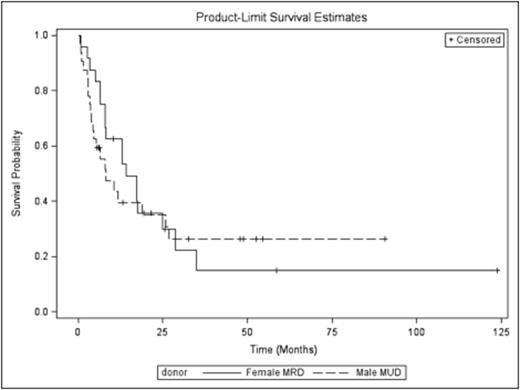
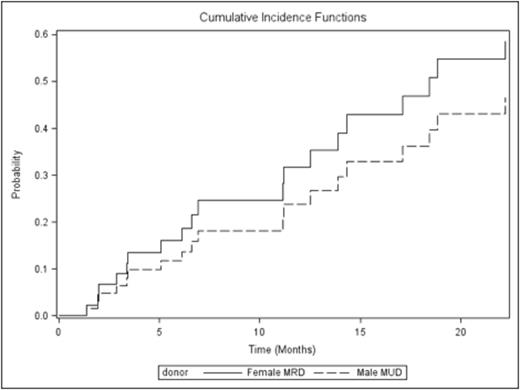
The median follow-up was 8 months (0.8-54 months). The median OS for patients who received M-MUD was 8.1 months while it was 14.2 months for patients who received F-MRD (p= 0.72) Fig1. Median DFS for patients who received M-MUD was 6 months while it was 7.2 months for patients who received F-MRD (p= 0.94). The CI rates of aGVHD III-IV, extensive chronic GVHD, REL and NRM at 12 months in M-MUD vs F-MRD were 13% vs 16% (p=0.69), 21% vs 27% (p=0.61), 38% vs 44% (P=0.65), and 16% vs 14% (p=0.79), respectively. The CI of SR GVHD in M-MUD vs F-MRD was 24% vs 32% (The Hazard Ratio of srGVHD in M-MUD vs F-MRD is 0.711 (95% confidence interval is 0.29-1.72, p=0.45)) Fig2. Other variables considered in univariate analysis for SR GVHD were recipient age (p=0.55), disease status (p= 0.64), MAC vs other regimens (p=0.58).
Conclusion: In this small cohort of consecutive patients from a single center, we found that compared to M-MUD, F-MRD had a trend for higher rates of SR GVHD after peripheral blood allo-SCT in adult male patients, although not statistically significant. There was no impact of F-MRD compared to M-MUD on OS, DFS, REL, NRM or rates of acute or chronic GVHD. However, the absence of statistical impact should be interpreted with caution given the retrospective design of the study and because we cannot exclude a possible limiting effect of a small number of patients.
Fig 1
Fig 2
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal