Abstract
Of late years, newly developed agents, such as bortezomib, lenalidomide, thalidomide, are widely used for treatment against multiple myeloma. After induction therapy, candidates for autologous stem cell transplantation are supposed to be followed by stem cell harvesting. There are several reports showing lenalidomide has a negative impact on stem cell mobilization. This opinion tends to let us refrain from using lenalidomide on the myeloma patients who are eligible for transplantation, even lenalidomide is expected promising. We experienced a series of three clinical cases presenting that stem cells were poorly mobilized with cyclophosphamide (CY) plus G-CSF after lenalidomide treatment, but sequential stem cell mobilization was incredibly improved with high-dose cytarabine plus G-CSF. One additional case who was treated with lenalidomide also presented successful stem cell mobilization with high-dose cytarabine plus G-CSF. Here we show all four cases in detail.
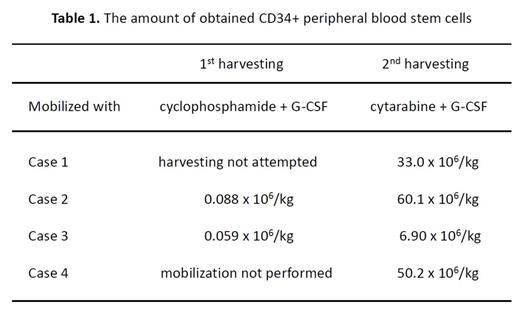
Case 1: 66 years old male, symptomatic myeloma after smoldering period. After three course of bortezomib induction, the response was insufficient. Sequentially he was treated with lenalidomide (25mg/day, every day for three weeks with one week rest period) and dexamethasone (Dex) (40mg/day, weekly) for two courses, and finally achieved Partial response (PR). First peripheral blood stem cell harvesting was attempted with high-dose CY (2.0 g/m2, day1-2) + lenograstim (5mg/kg daily, on days 7 until leukapheresis), but mobilization was unsuccessful so harvesting was not performed. For subsequent mobilization, high-dose cytarabine was administered at a dose of 2.0 g/m2 twice daily (day1-2) + lenograstim. Second mobilization was markedly improved, and finally 33.0 x 106/kg CD34+ cells were obtained.
Case 2: 63 years old male, symptomatic myeloma, IgG type. This patient was treated with bortezomib, CY and Dex but resulted in disease progression. As an alternative therapy, lenalidomide (10mg/day, daily for three weeks with one week rest) and Dex (40mg, weekly) were used for three cycles. The dose of lenalidomide was reduced due to renal dysfunction. PR was obtained, then first harvesting was attempted with high-dose CY + lenograstim, as case 1, and 0.088 x 106/kg of CD34+ cells were collected, which was not sufficient for transplantation. Second mobilization was performed with high-dose cytarabine as case 1, and consequently we could obtain 60.1 x 106/kg of CD34+ cells; the yield was dramatically improved.
Case 3: 41 years old female, symptomatic myeloma after one year course of smoldering myeloma. As an induction therapy, bortezomib, CY and Dex were selected, but finally she could not achieved PR after three cycles. We gave up bortezomib-based induction, and then lenalidomide (15-25 mg on day1-21 with 1 week rest) and Dex (40 mg, weekly) were administrated for five courses, followed by PR. As previous two cases, the first peripheral stem cell collection was initiated with high-dose CY + lenograstim, and it was not sufficient (0.059 x 106/kg of CD34+ cells). And the second mobilization with high-dose cytarabine with lenograstim recovered the yield of stem cell up to 6.90 x 106/kg.
Case 4: 63 years old male, symptomatic myeloma. He was treated with bortezomib, Dex with/without CY, but this regimen was not very effectual, and CY caused elevation of aminotransferase (CTCAE grade 3). Then lenalidomide (10-15 mg on day 1-21) and Dex (40 mg, weekly) were administrated for four courses, and the patient achieved PR. Due to the adverse effect of liver dysfunction, we could not use high-dose CY for mobilization. For this case, high-dose cytarabine was selected for first mobilization, and it was very successful, 50.2 x 106/kg of CD34+ cells were harvested. The yields of PBSC from all four cases are summarized on Table 1.
These four cases suggest mobilization with high-dose cytarabine could be an alternative option for poor mobilizer of myeloma patient treated with lenalidomide-based induction. This fact may enable us to choose lenalidomide, not only bortezomib, for induction even for transplant-eligible cases.
Ishiko:Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal