Abstract
The pharmacokinetic challenges of warfarin therapy have led to the development of new oral anticoagulants (NOACs) that directly inhibit coagulation factor Xa (FXa). While these new drugs (including rivaroxaban and apixaban) have many benefits over warfarin, no approved strategy exists to reverse their anticoagulant effects in the event of life-threatening bleeding or emergent need for surgery. The most promising reversal strategy in clinical development is a catalytically inactive form of FXa (Gla-domainless FXaS195A, GD-FXaS195A) that binds the NOAC with high affinity to relieve inhibition of endogenous FXa. In principle, removing the inhibitor through molecular engagement is attractive, especially when administered before bleeding begins (e.g. pre-surgery), and there is in vivo evidence to support the efficacy of GD-FXaS195A as a pre-injury antidote for direct FXa inhibitors. However, since GD-FXaS195A is a scavenger of the inhibitor and not a pro-hemostatic agent, GD-FXaS195A may not be effective if given after a bleeding episode has begun. Moreover, its mechanism of action necessitates a 1:1 ratio of GD-FXaS195A to neutralize the inhibitor. Thus, high doses (hundreds of milligrams of protein) will likely be required in humans.
We hypothesized that a pro-hemostatic bypassing agent might be able to reverse the effects of direct FXa inhibitors no matter when it is administered, and with high potency. To evaluate this, we used a variant of FXa (FXaI16L) that is more zymogen-like than wild-type (wt)-FXa. This "zymogen-like" variant has lower catalytic activity in in vitro assays compared to wt-FXa due to impaired active site maturation. It is also resistant to active site inhibitors including plasma protease inhibitors, resulting in an extension of its plasma half-life (>30 minutes vs ~1 minute for wt-FXa). Importantly, its activity is rescued upon binding to its cofactor FVa in vivo, and we have previously shown that FXaI16L bypasses the intrinsic pathway defect in hemophilic mice. Here we evaluated whether FXaI16L might also be able to bypass direct FXa inhibitors using in vitro thrombin generation assays (TGAs) and two in vivo injury models in mice, and directly compared FXaI16L to GD-FXaS195A. In TGA experiments, 500 nM rivaroxaban decreased peak thrombin generation to 23% of that observed in normal human plasma (NHP). This decreased thrombin generation could be reversed by the addition of 3 nM human (h)FXaI16L which normalized peak thrombin generation. In comparative studies, hFXaI16L was 300-fold more potent than GD-FXaS195A in TGA assays.
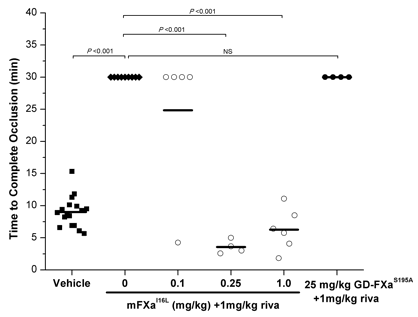
These data highlight that differences in mechanism of action (pro-hemostatic vs. scavenger) will have a huge impact on amounts of protein needed to revive thrombin generation. This was further reflected in in vivo studies. In invivo experiments with wild-type mice using 7.5% FeCl3 to induce carotid artery thrombosis, 1 mg/kg rivaroxaban prevented formation of occlusive thrombi. 30 minutes after the initial FeCl3 injury to rivaroxaban-treated mice, infusion of 0.25 mg/kg murine (m)FXaI16L normalized the phenotype, causing rapid occlusion at the injury site within 5 minutes. In contrast, we did not observe carotid artery occlusion with administration of up to 25 mg/kg GD-FXaS195A 30 minutes after the injury to rivaroxaban-treated mice (Fig. 1). When both rivaroxaban and the reversal agent were given prior to the injury, both mFXaI16L (0.5 mg/kg) and GD-FXaS195A (25 mg/kg) were effective at reversing the inhibition. Furthermore, using intravital microscopy to examine thrombus formation at the site of laser injury to mouse cremasteric arterioles, we found that 1 mg/kg rivaroxaban completely abrogated fibrin deposition and substantially reduced platelet accumulation. Treatment with1 mg/kg mFXaI16L substantially increased platelet and fibrin deposition at the site of laser injury.
Carotid occlusion time when the reversal agent was infused 30 minutes after the FeCl3 injury.
Carotid occlusion time when the reversal agent was infused 30 minutes after the FeCl3 injury.
Taken together, these data provide strong support for the in vivo efficacy and potency of FXaI16L as a potential pro-hemostatic bypass agent to reverse direct FXa inhibitors. The data also illustrate that antidotes like GD-FXaS195A may not be as effective as pro-hemostatic agents like FXaI16L following an injury, and we predict that effective reversal of NOACs will require a nuanced approach that uses an antidote or a bypassing agent depending on the clinical scenario.
Jasuja:Pfizer: Employment. Patel-Hett:Pfizer: Employment. Fruebis:Pfizer: Employment. Pittman:Pfizer: Employment. Camire:Pfizer: Consultancy, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal