Abstract
Introduction: The introduction of novel therapies has revolutionized the treatment of multiple myeloma in the last 20 years. Outcomes vary significantly by age with younger patients experiencing longer survival than older patients. The majority of cancer patients in the United States receive treatment in community oncology practices, as opposed to university hospital settings. RainTree Analytics maintains a unique database of electronic medical record (EMR) data from RainTree Oncology member community oncology practices. We sought to understand the treatment patterns and patient demographics of multiple myeloma patients in community oncology practices.
Methods: A search of the RainTree Analytics database was conducted for patients with an ICD-9 code of 203.0-203.02 (multiple myeloma). 2771 patients who received treatment for multiple myeloma between January 2, 2012 and July 9, 2014 were identified. Of these 2022 had received either bortezomib or lenalidomide, or both. We compared the characteristics of patients who had received prescriptions for bortezomib but not lenalidomide (N = 551) or lenalidomide but not bortezomib (N = 699), the two most frequently prescribed multiple myeloma therapies, excluding melphalan and dexamethasone.
Results: Most patients who received either bortezomib or lenalidomide, but not both, were over 60. The age distribution of these two populations is provided in Table 1.
Age Distribution of Bortezomib and Lenalidomide Patients
| AGE . | Bortezomib Patient Count (%) . | Lenalidomide Patient Count (%) . |
|---|---|---|
| 21-30 | 1 (0.18) | 1 (0.14) |
| 31-40 | 4 (0.73) | 2 (0.29) |
| 41-50 | 17 (3.09) | 38 (5.44) |
| 51-60 | 65 (11.80) | 107 (15.31) |
| 61-70 | 147 (26.68) | 220 (31.47) |
| 70+ | 317 (57.53) | 331 (47.35) |
| Total | 551 | 699 |
| AGE . | Bortezomib Patient Count (%) . | Lenalidomide Patient Count (%) . |
|---|---|---|
| 21-30 | 1 (0.18) | 1 (0.14) |
| 31-40 | 4 (0.73) | 2 (0.29) |
| 41-50 | 17 (3.09) | 38 (5.44) |
| 51-60 | 65 (11.80) | 107 (15.31) |
| 61-70 | 147 (26.68) | 220 (31.47) |
| 70+ | 317 (57.53) | 331 (47.35) |
| Total | 551 | 699 |
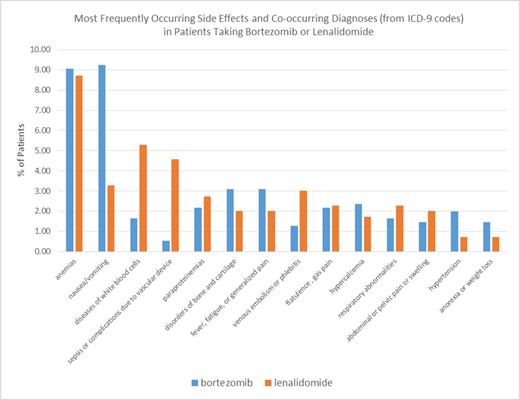
Side effects and co-occurring diagnoses listed as ICD-9 codes in the EMR were analysed. The most frequently co-occurring diagnoses reported in the EMR in bortezomib and lenalidomide patients were anemias, nausea and vomiting, and diseases of white blood cells (Figure 1). Anemias were reported at approximately the same frequency in bortezomib and lenalidomide patients, 9.07% and 8.73%, respectively. Nausea and vomiting (ICD-9 codes 787.01-787.2) were reported more frequently in bortezomib patients than in lenalidomide patients, 9.26% and 3.29%, respectively, but diseases of white blood cells (1.63% (bortezomib) and 5.29% (lenalidomide)) and sepsis or complications due to vascular devices (0.54% (bortezomib) and 4.58% (lenalidomide)) were reported more frequently in lenalidomide patients.
Side Effects and Co-occurring Diagnoses in Patients Taking Bortezomib and Lenalidomide
Side Effects and Co-occurring Diagnoses in Patients Taking Bortezomib and Lenalidomide
Bortezomib and lenalidomide duration of therapy were analysed for patients who had received at least one refill for the therapy. The mean duration of therapy for bortezomib and lenalidomide was 203 and 250 days, respectively (Table 2). The mean number of prescriptions written for bortezomib was greater than the mean number of prescriptions written for lenalidomide, as would be expected for the methods of delivery of the two therapies.
Duration of Bortezomib or Lenalidomide Therapy
| . | Bortezomib . | Lenalidomide . | |
|---|---|---|---|
| Mean Number of Prescriptions Written | 20 | 9 | |
| Duration of Therapy | |||
| Mean | 203 | 250 | |
| Median | 142 | 163 | |
| Minimum | 33 | 30 | |
| Maximum | 903 | 908 | |
| Standard Deviation | 178 | 224 | |
| . | Bortezomib . | Lenalidomide . | |
|---|---|---|---|
| Mean Number of Prescriptions Written | 20 | 9 | |
| Duration of Therapy | |||
| Mean | 203 | 250 | |
| Median | 142 | 163 | |
| Minimum | 33 | 30 | |
| Maximum | 903 | 908 | |
| Standard Deviation | 178 | 224 | |
Conclusions: The age distributions and duration of therapy for bortezomib and lenalidomide were similar for the two therapies, however, there were significant differences in co-occurring diagnoses reported by ICD-9 in the EMR. Further study is warranted to investigate which of these co-occurring diagnoses are associated with pre-existing patient characteristics and which may be the results of differences in therapies.
Reichert:RainTree Oncology Services: Employment. Patton:Pfizer: Consultancy; Johnsone & Johnson: Consultancy; Tasaro: Consultancy; Bristol Myers Squibb: Consultancy; Lilly: Consultancy; Genentech: Consultancy; Amgen: Consultancy; RainTree Oncology Services: Employment; Astellas: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal