Abstract
Multiple myeloma (MM) is a clonal disease characterized by an increased number of malignant plasma cells (M-PCs) that accumulate in bone marrow (BM). High dose of chemiotherapy followed by autologous peripheral blood stem cell transplantation produces a high rate of complete remissions; however the duration of such responses does not usually exceed three years due to the persistence of residual tumor cells, known as minimal residual disease (MRD), responsible for tumor relapse. The aetiology of malignancy remains unknown, although the BM microenvironment plays a critical role in the genesis by stimulating the proliferation of M-PCs and inhibiting the apoptosis.
The aim of this study is to analyse and compare the phenotype of M-PCs with that of normal PCs as well as to correlate it with clinical outcome of MM patients in order to identify significant phenotypic differences able to 1) contribute to the differential diagnosis of monoclonal gammopathies by clearly identifying and characterizing M-PCs; 2) identify new prognostic markers; 3) evaluate MRD and monitor treatment efficacy; 4) identify new potential specific markers of M-PCs suggesting antibody-based targeted therapies.
A total of 45 patients (pts) were enrolled in the study and their BM samples collected at the diagnosis: 15 patients with active MM, 10 with non-active MM, 10 with MGUS and 10 controls. The BM samples of MM patients were prospectively collected following each course of therapy, and in particular at time +100 after HDT/ASCT, as well as at the time of relapse or progression. At each consultation a multiparametric flow cytometric immunophenotype evaluation was performed using the following monoclonal antibodies: CD56, CD19, CD138 (CD38), CD45 and immunoglobulin κ and λ light chains to identify PCs . The CD117, CD40 and CD27 antibodies were used to distinguish distinct populations of PCs. A direct 8-color method was applied to study the immunophenotype of PCs, utilizing a Navios Cytometer (Beckaman Coulter), and analysing with Kaluza software. Immunophenotype data were compared with them of immunofixation (IMF) and polymerase chain reaction (PCR) approaches. The study was approved by the local Ethics Committee and all patients provided their informed consent in accordance with the Declaration of Helsinki.
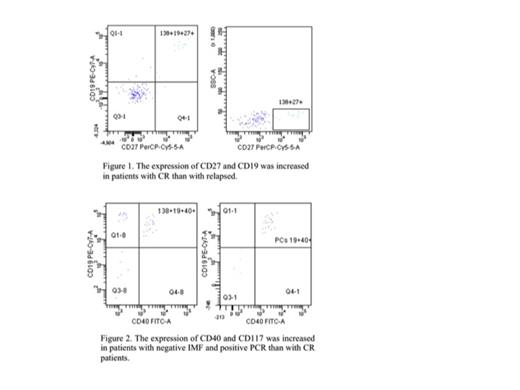
Significant immunophenotype changes were found in the marrow infiltration of M-PCs, and, mainly, in the aberrant expression of CD27 and CD40/CD117. Almost all patients (93,3%) with active MM showed the characteristic immunophenotype of M-PCs with aberrant expression of CD56 and concomitantly lack of CD19 and CD27. When the expression of CD27 was evaluated in M-PCs following the therapy and compared with both IMF and PCR data seemed to be modulated by disease relapse/progression or disease treatment (fig.1) resulting a significant increased (P=0.006) in pts with complete response (CR) (IMF and PCR negative) as compared with them relapsed and lost with myeloma progression. An inverse correlation was observed by evaluating the expression of CD40 and CD117 that seemed be modulated by disease treatment. Following the therapy the simultaneous expression of CD40/CD117 was observed in almost pts (86%) with negative IMF and positive PCR. Conversely this characteristic immunophenotype fingerprint was absent in all pts with CR. However the expression of CD117 in CD38+CD138+ cells was greater in MGUS or non-active MM pts than in pts in CR (p<0,01).
In the present study, we assumed that the contribution of different antigens assessed simultaneously would give more information to diagnosis of MM pts and to better stratify for risk of relapse/progression in order to initiate the most appropriate treatment, and to monitor treatment efficacy. CD19 and CD27 together resulted in optimal accordance with IMF and PCR approaches. Conversely CD40/CD117 showed a good accordance with disease response to the treatment. Our next goal will be to enrol more patients in order to generate a model of forecasting that evaluates simultaneous all three antigens assigning each one a specific weight in the contribution of diagnosis/prognosis. This will be useful to identify a immunophenotype fingerprint on which to design antibody-based targeted therapies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal