Abstract
In a retrospective cohort, seven patients with multiple myeloma carrying the BRAF V600E mutation had significantly shorter overall survival and higher prevalence of extramedullary disease than 251 BRAF wild type (wt) controls (Andrulis et al Cancer Discov 2013). Three case reports are in concordance with this view (Bohn et al and Sharman et al, Clin Lymphoma Myeloma Leuk, 2014). We conducted this study to further investigate the clinical and biological implications of this mutation in multiple myeloma.
We used mutation-specific quantitative real-time PCR (qPCR) to screen biopsies from 209 myeloma patients, of which 185 were taken prior to first relapse. The BRAF V600E mutation was detected in 13 (6.2 %) of the patients. 10 of them also expressed the corresponding protein as evaluated by immunohistochemistry (IHC) using the BRAF V600E specific VE1 antibody, and 2 patients had simultaneous mutations in BRAF and NRAS/KRAS. RAS mutations are of particular interest because in vitro studies indicate that their presence may imply a paradoxical effect of BRAF inhibitors (Lohr et al Cancer Cell 2014).
Whole exome sequencing (WES) was carried out in three BRAF V600E positive patients from whom we had stored purified myeloma cells. Among the top 11 recurrently mutated genes listed in Lohr et al (Cancer Cell 2014), we found mutations only in BRAF, NRAS, and KRAS. Clonal fraction of BRAF V600E mutated plasma cells as evaluated by IHC and WES varied from 4 to 100 %. There was agreement between detection methods in the patient with a dominating V600E mutated clone, but less so in the patients with small clones.
Estimates of BRAF V600E clone size and RAS mutation analysis in 13 patients positive for BRAF V600E by qPCR
| IHC (clone size, %) . | WES (clone size, %) . | Sanger sequencing . | NRAS / KRAS mutation . |
|---|---|---|---|
| 75-100 | 86 | + | negative |
| 75-100 | negative | negative | |
| 75-100 | + | negative | |
| 50-75 | + | negative | |
| 50-75 | + | negative | |
| 25-50 | negative | negative | |
| 25-50 | negative | negative | |
| <25 | 0 | negative | KRAS p.G12A (ex2) and p.Q61H (ex3) |
| <25 | + | negative | |
| <25 | + | negative | |
| negative | negative | negative | |
| negative | 4 | negative | NRAS p.Q61K (ex3) |
| negative | negative | negative |
| IHC (clone size, %) . | WES (clone size, %) . | Sanger sequencing . | NRAS / KRAS mutation . |
|---|---|---|---|
| 75-100 | 86 | + | negative |
| 75-100 | negative | negative | |
| 75-100 | + | negative | |
| 50-75 | + | negative | |
| 50-75 | + | negative | |
| 25-50 | negative | negative | |
| 25-50 | negative | negative | |
| <25 | 0 | negative | KRAS p.G12A (ex2) and p.Q61H (ex3) |
| <25 | + | negative | |
| <25 | + | negative | |
| negative | negative | negative | |
| negative | 4 | negative | NRAS p.Q61K (ex3) |
| negative | negative | negative |
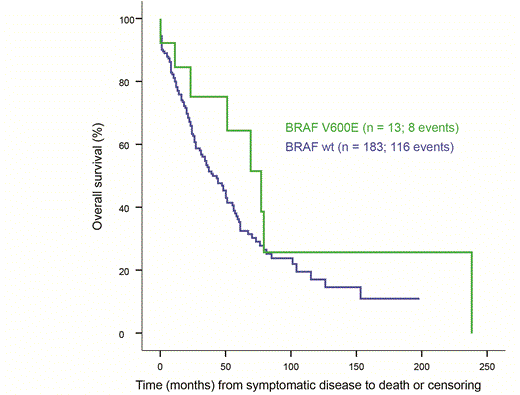
BRAF V600E positive patients presented a variety of clinical phenotypes and no characteristic genotype-phenotype relation could be identified. Progression free and overall survival, and all clinical parameters including the presence of extramedullary disease, were similar in patients with BRAF V600E and BRAF wt. Three patients carried the mutation in > 75 % of tumour cells, and all had indolent disease. One of them died after seven years, whereas the remaining two responded with long lasting CR to broad acting therapy (MP, MPT) and are still in remission after five and seven years. The Regional Ethics Committee approved the study (REK 2165-2012).
Overall Survival from symptomatic disease
In conclusion, we found no evidence supporting a prognostic role or association with a particular clinical phenotype for the BRAF V600E mutation in early multiple myeloma, which is in contrast to earlier reports. Furthermore, the three patients with a high fraction of mutated cells had a relatively benign disease responsive to conventional treatment. This study demonstrates that the role of mutation V600E is more diverse than previously assumed. Presence, and particularly high fraction of BRAF V600E mutated cells as well as translation to protein, are prerequisites for specific inhibitory treatment. However, these characteristics are alone not sufficient to predict outcome or to choose the right treatment.
Hovig:PubGene Inc.: Equity Ownership; GeneSeque AS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Vodák:PubGene AS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal