Abstract
Background: Evidence-based practices have shown that transfusion program (TP) is beneficial to SCA-patients with abnormally high velocities by Doppler; however, TP cannot be stopped safely, except following HSCT. No prospective trial has to date compared the extent of cerebral vasculopathy following TP or HSCT. The premise of the French National Trial “Drepagreffe” is that cerebral velocities will be reduced to a greater extent after HSCT than under TP.
Patients and Methods: We present here preliminary results from this prospective trial with 2 arms (TP/HSCT), defined by the random-availability of a genoidentical donor. Inclusion criteria were SCA (SS/Sb0) children younger than 15 years with a history of abnormal cerebral arterial velocities (TAMMX ≥ 200 cm/sec), placed on long-term transfusion programs, with at least one non-SCA sibling and parents accepting HLA-typing and HSCT if a genoidentical donor was available. Transplanted patients received as conditioning regimen Busilvex-CY 200 mg/kg and 20 mg/kg rabbit Thymoglobulin with CSA and short MTX or MMF for GVHD prophylaxis. In the TP arm, HbS% was maintained at < 30% with Hb 9-11g/dL. At enrollment and 12 months post-enrollment, blood screening, Doppler, cerebral MRI/MRA were performed along with cognitive performance testing, the latter done in parallel in the control sibling. Primary endpoint was the significantly greater reduction of velocities in the HSCT than in the TP arm. Among the various secondary endpoints, Doppler normalization defined by velocities < 170 cm/s in all arteries was to occur more often after HSCT than on TP.
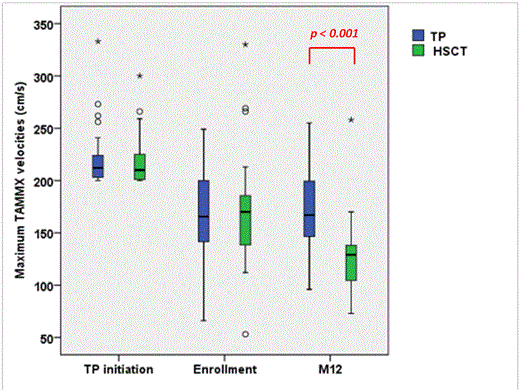
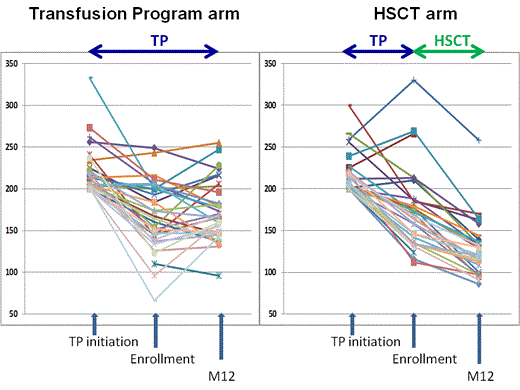
Results: SCA-children (n=67; 36F-31M) from 10 French SCA-centers were enrolled between 12/2010 and 6/2013 at the mean (SD) age of 7.6 (3.1) years. History of stroke was present in 6 patients (4 in HSCT and 2 in TP) and 1 TIA in HSCT arm. At TP initiation, velocities≥200/cm/sec were found in middle (n=50), anterior (n=11) and internal carotid arteries (n=30) as abnormal velocities were observed in more than one artery in several patients. Mean (SD) maximum velocities were 219 (26) cm/s (range: 200-333). At enrollment all patients were on TP and paired analysis showed that mean(SD) maximum velocities had significantly decreased (p<0.001) under TP:169 (46) cm/s vs 219 (26) cm/s). Following HLA-typing, 35 without genoidentical donor were included in the transfusion arm and 32 with genoidentical donor were transplanted in 6 HSCT-centers. Mean (SD) maximum velocities were not significantly different in both arms at enrollment: 167 (41) in TP vs 170 (51) cm/s in HSCT. During the 12 months follow-up, no stroke was observed but one patient in the TP arm experienced a hyperammonemic reversible coma, without MRI/MRA alteration requiring transfer to intensive care. In the HSCT arm, all patients successfully engrafted, one grade II and two grade III acute GVHD, and no chronic GVHD were observed. Two patients required transfer to intensive care for seizures and pneumonia. Other complications were seizures (n=2), CMV (n=9) or EBV replications (n=1), hemorrhagic cystitis (n=3), aspergillosis (n=1), prolonged but reversible thrombopenia (n=2), transitory hemolytic anemia (n=1). At 12 months, data, available in 63/67 patients, showed that all patients were alive, mean (SD) Hb and HbS% in TP arm were 9.1 (0.9) and 27.5% (11.9), respectively, whereas in the HSCT arm, mean (SD) Hb and % donor chimerism were 12.0 (1.0) g/dL and 86.5% (12.2) respectively (range:60-100%). All transplanted patients had the same Hb electrophoresis than their donor. Mean (SD) maximum velocities were significantly lower post-HSCT (n=31) than under TP (n=32):128 (34) vs 174 (36) cm/s, respectively; (p<0.001), and were decreased more significantly following HSCT than on TP: mean(SD)Δ: -44 (24) vs +6 (3), respectively. The percentage of patients with normal velocities was significantly higher post-HSCT (27/31) than in the TP arm (16/32) (p=0.003).
Conclusions: This prospective national trial comparing TP vs. HSCT in SCA-patients with a history of abnormal velocities shows for the first time that HSCT repeatedly and significantly results in a greater decrease in velocities than TP, and has very little toxicity. These preliminary results are encouraging and suggest that suppression of host SCA-erythropoiesis by HSCT is the treatment of choice for SCA-children with abnormal-TCD and genoidentical donor.
Bernaudin:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal