Abstract
The international prognostic scoring system (IPSS) for MDS patients (pts) receiving supportive care predicts a median overall survival of 68.4 months (mo), 42 mo, 14.4 mo and 4.8 mo according to the subgroups low, Int-1, Int-2, and high-risk, respectively. In July 2009, we established the German outpatient MDS registry with the aim to describe diagnostic procedures, treatment and course of disease of MDS pts in the community outpatient setting. In this analysis we evaluate the incidence of the IPSS subgroups, the frequencies of different therapies and the outcome, respectively.
Methods: Newly diagnosed pts with MDS confirmed by bone marrow biopsy who gave informed consent were registered in an online database (IoStudy®). Baseline characteristics and diagnostics as well as a quarterly update of the course of disease were documented.
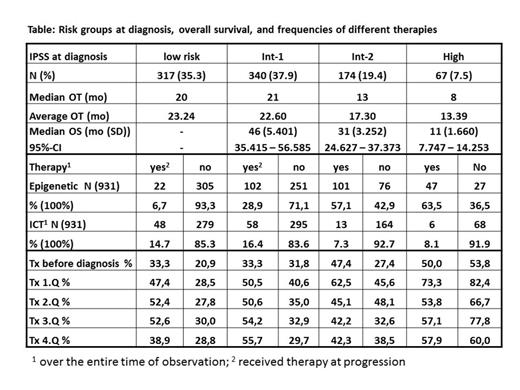
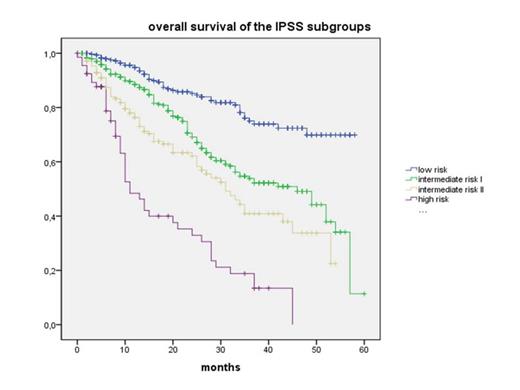
Results: From July 2009 to March 2014 (56 mo) 1368 pts from 78 institutions were registered. Mean age was 73.5 years (y) (min-max 26.5 - 94.3 y, median 74.5 y) and 41% were women. 1,262 pts (92.3%) were evaluable for follow-up with a mean observation time (OT) of 21.7 mo (median: 19 mo). At diagnosis, IPSS risk classification was available in 898/1,262 (71%) pts. OT, overall survival (OS), number of pts (N) with epigenetic and iron-chelation therapy (ICT) over the entire term and the need of transfusions (tx) per quarter (Q) are given according to risk groups and treatment in the table. The figure shows the estimated Kaplan-Meyer-Plot for the IPSS risk groups. 180/1,315 pts (13.7%) received ICT, whereas 1,035 did not (86.3%). Median OS was 51 (95% confidential interval (CI) 44.7-57.3) and 49 mo (95% CI 43.7-54.3) (p = 0,281) for those with and without ICT, respectively.
Conclusion: In the community outpatient setting in Germany, epigenetic therapy is used in a relative high proportion of pts with int-2 risk (57.1%) and high risk MDS (63.5%) at diagnosis, as well as in a substantial minority of patients with low-risk (6.7%) and int-1 risk (28.9%) MDS at progression. In the higher risk groups, transfusion need decreased more than 10% over time and overall survival was substantially longer than predicted by the IPSS. This may be considered as an empirical evidence for the efficacy of the new therapeutic options in regular care.
Supported by Celgene, Munich, and Novartis, Erlangen, Germany
Steinmetz:Novartis: Consultancy, Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Severin:Novartis: Research Funding; Celgene: Research Funding. Germing:Celgene: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Schmitz:Novartis: Consultancy, Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau. Gattermann:Celgene: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal