Abstract
BACKGROUND: Lymphomas are the 5th most common cancer in the U.S. and most are incurable with standard therapy. Previously, we completed three trials of ‘in situ vaccination’ - combining low-dose radiotherapy (XRT) with intratumoral administration of TLR9 agonist (CpG). We demonstrated induction of anti-tumor CD8 T cell responses and clinical remissions of patients’ non-irradiated sites of disease, lasting up to 4+ years. One limitation may have been the paucity of intratumoral dendritic cells (DC). DC are uniquely able to endocytose dying (e.g. irradiated) tumor cells for cross-presentation to anti-tumor CD8 T cells.
METHODS: Flt3L– the predominant DC differentiation factor– induces tumor leukocyte infiltration and regression of lymphoma tumors pre-clinically and a new formulation of this cytokine -CDX-301- was shown to mobilize BDCA-1 and BDCA-3 DC subsets in an early phase trial. These DC subsets respond to several TLR agonists and cross-present antigens more effectively than plasmacytoid DC (the CpG-responsive DC subset). We initiated a phase I/II study of a new iteration of the in situ vaccine, adding Flt3L-priming and replacing the prior TLR9 agonist with the TLR3 agonist poly-ICLC (Fig 1A). The vaccine consists of:
-intratumoral Flt3L administration to increase DC within the tumor
-low-dose XRT to induce immunogenic tumor cell death and release tumor-associated antigens, and
-intratumoral poly-ICLC administration to activate tumor antigen-loaded DC.
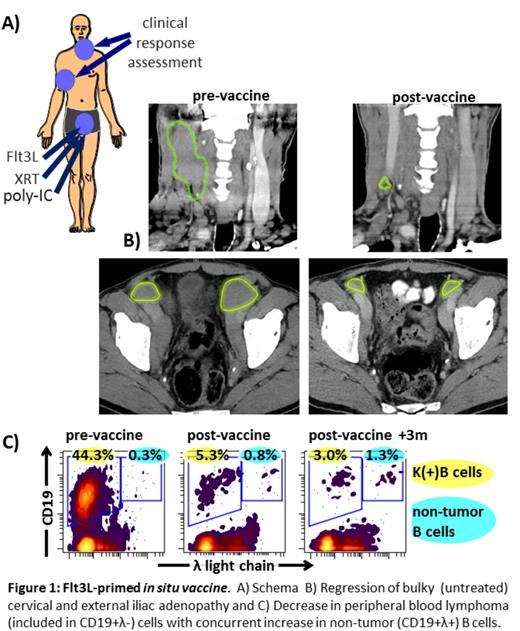
RESULTS: Six patients have been enrolled, two patients have completed therapy. Treated patients had 2-200-fold increases in BDCA1 and BDCA3 intratumoral DC after Flt3L administration and marked DC activation after XRT and poly-ICLC. Both treated patients have had partial remissions of untreated sites per Cheson criteria, persisting or improving for >6 months after vaccination. These include regressions of bulky lymph nodes (Fig 1B), as well as peripheral blood (Fig1C) and bone marrow disease. A patient with significant peripheral blood tumor burden experienced >10-fold decrease in malignant B cells with concurrent increase in non-tumor B cells, suggesting a degree of cell specificity in the tumor-killing mechanism. Adverse effects have been mild.
CONCLUSIONS: Preliminary results suggest that the Flt3L-primed in situ vaccine is feasible, safe and immunologically and clinically effective, warranting further study.
Off Label Use: CDX-301 - the purpose of which is to mobilize dendritic cells to an injected tumor site.. Crowley:Celldex Therapeutics Inc: Employment. Keler:Celldex Therapeutics, Inc.: Employment. Davis:Celldex Therapeutics Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal