Abstract
Background:
An error at the drug compounder source resulted in accidental over-dilution of the chemotherapy drugs cyclophosphamide and gemcitabine utilized by several cancer centers in Ontario and New Brunswick in Canada between March 2012 and March 2013. The exact degree of unintentional dose reduction for each patient is unknown, but the estimate is between 3-20% given the type of dilution error. One thousand two-hundred and two cancer patients were affected overall, including 177 hematology patients at London Regional Cancer Center (LRCP). We evaluated the effect of cyclophosphamide under-dosing on patients with diffuse large B cell lymphoma (DLBCL) treated with chemotherapy containing cyclophosphamide (mostly R-CHOP), the largest subset of patients affected.
Methods:
We conducted a retrospective cohort study of all DLBCL patients at LRCP who received at least one cycle of chemotherapy containing diluted cyclophosphamide between March 2012 and March 2013. The affected cohort was compared to a historical group of DLBCL patients matched by stage (early versus advanced) and age (±5 years) who were treated prior to the dilution error from January 2008 to December 2010. The main study outcome was event-free survival defined as a composite of disease progression or death. Groups were compared using unpaired Student’s t-test, Chi-squared or Fisher’s exact tests, as appropriate. Survival analysis was done using the Kaplan-Meier method.
Results:
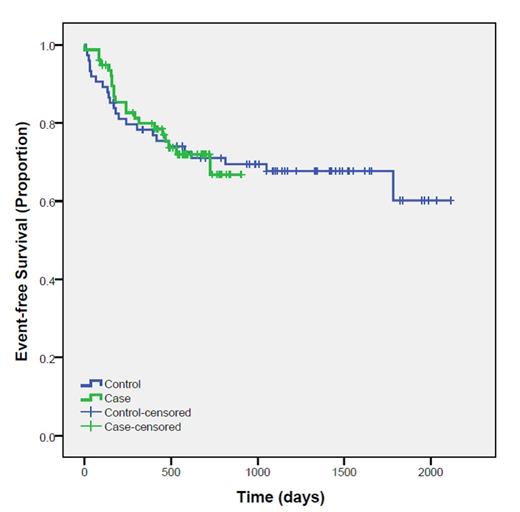
Seventy-seven patients received diluted cyclophosphamide and were matched to 74 historical controls. There were no differences in the baseline characteristics between groups (Table 1). Complete remission was achieved in 41 (53.2%) cases and 43 (58.1%) controls (p= 0.55). Overall response rate was 71.2% in cases and 67.6% in controls. At a median follow up of 490 days, progression or death occurred in 21 (27.3%) cases and 24 (32%) controls (P= 0.49). Survival analysis of the main outcomes showed no difference between the two study groups (log rank 0.999).
Conclusion:
There is no difference in the rate of response or in event-free survival among DLBCL patients receiving one or more doses of accidentally diluted cyclophosphamide, compared to a matched historical control group. This suggests that a minor dose reduction in 1 drug in multi-agent chemotherapy may be inconsequential in terms of disease response and risk of relapse.
Baseline Characteristics
| Variable . | Cases N=77 . | Control N= 74 . | P value . |
|---|---|---|---|
| Male [N (%)] | 39 (52) | 36 (48) | 0.87 |
| Age (Mean) Interquartile range | 77 (SD ± 16) 55-79 | 66 (SD±14) 56 - 77 | 0.665 |
| Age > 60 [N (%)] | 29 (37.7) | 24 (32.4) | 0.5 |
| LDH [Mean (SD)] | 408 (375) | 394 (50) | 0.819 |
| WBC [Mean (SD)] | 8.2 (4) | 7.5 (3) | 0.193 |
| Hemoglobin[Mean (SD)] | 117 (22) | 120 (21) | 0.348 |
| Platelets [Mean (SD)] | 259 (175) | 253 (128) | 0.82 |
| Extra-nodal > 1 [N (%)] | 18 (23.4) | 18 (24.3) | 0.89 |
| Stage III/IV [N (%)] | 53 (68.9) | 51 (68.8) | 0.99 |
| B-symptoms [N (%)] | 44 (57.1) | 43 (49.4) | 0.83 |
| ECOG ≥ 2 [N (%)] | 33 (42.9) | 36 (49.3) | 0.4 |
| High LDH [N (%)] | 52 (67.5) | 43 (58.1) | 0.23 |
| High risk (R-IPI of score ≥3) [N (%)] | 27 (35.1) | 28 (37.8) | 0.5 |
| Prescribed (intentional) dose reduction [N (%)] | 23 (30) | 25 (34.7) | 0.52 |
| Radiation therapy [N (%)] | 33 (42.9) | 34 (46.6) | 0.53 |
| Febrile neutropenia [N (%)] | 21 (27.3) | 15 (20.3) | 0.31 |
| GCSF use [N (%)] | 48 (62.3) | 38 (53.5) | 0.28 |
| Variable . | Cases N=77 . | Control N= 74 . | P value . |
|---|---|---|---|
| Male [N (%)] | 39 (52) | 36 (48) | 0.87 |
| Age (Mean) Interquartile range | 77 (SD ± 16) 55-79 | 66 (SD±14) 56 - 77 | 0.665 |
| Age > 60 [N (%)] | 29 (37.7) | 24 (32.4) | 0.5 |
| LDH [Mean (SD)] | 408 (375) | 394 (50) | 0.819 |
| WBC [Mean (SD)] | 8.2 (4) | 7.5 (3) | 0.193 |
| Hemoglobin[Mean (SD)] | 117 (22) | 120 (21) | 0.348 |
| Platelets [Mean (SD)] | 259 (175) | 253 (128) | 0.82 |
| Extra-nodal > 1 [N (%)] | 18 (23.4) | 18 (24.3) | 0.89 |
| Stage III/IV [N (%)] | 53 (68.9) | 51 (68.8) | 0.99 |
| B-symptoms [N (%)] | 44 (57.1) | 43 (49.4) | 0.83 |
| ECOG ≥ 2 [N (%)] | 33 (42.9) | 36 (49.3) | 0.4 |
| High LDH [N (%)] | 52 (67.5) | 43 (58.1) | 0.23 |
| High risk (R-IPI of score ≥3) [N (%)] | 27 (35.1) | 28 (37.8) | 0.5 |
| Prescribed (intentional) dose reduction [N (%)] | 23 (30) | 25 (34.7) | 0.52 |
| Radiation therapy [N (%)] | 33 (42.9) | 34 (46.6) | 0.53 |
| Febrile neutropenia [N (%)] | 21 (27.3) | 15 (20.3) | 0.31 |
| GCSF use [N (%)] | 48 (62.3) | 38 (53.5) | 0.28 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal