Abstract
INTRODUCTION. Desirudin is a recombinant direct thrombin inhibitor (DTI) approved in the US for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in elective hip replacement surgery, and approved in European Union for the prevention of DVT in elective hip or knee replacement surgery. A multicenter, double-blind, randomized, controlled trial comparing the safety and efficacy of desirudin with enoxaparin in elective total hip replacement (THR) surgery demonstrated that desirudin has a safety profile similar to enoxaparin but is more effective in preventing both overall and proximal DVT (Eriksson et al, 1997). The objective of this study was to conduct post hoc analyses of the efficacy of desirudin versus enoxaparin in high-risk patient subgroups.
METHODS. Data were collected from 2079 patients randomized at medical centers in 10 European countries. Desirudin was administered 15 mg subcutaneously (SC) twice daily (BID) on the day of the surgery, once within 30 minutes prior to surgery and once in the evening, and 15 mg BID thereafter, postoperatively (n=1043). Enoxaparin was administered 40 mg SC once daily (QD) on the day prior to surgery (12 hours preoperatively), then at the same dose on the day of the surgery and QD thereafter, postoperatively for 9-12 days. The primary outcome was major thromboembolic event (proximal DVT, PE, or death) during the prophylaxis period; secondary outcomes were any thromboembolic event (proximal and distal DVT, PE, or death) during this same period. Confirmatory venography was performed if clinical symptoms occurred. Subsequent post hoc efficacy analyses for desirudin versus enoxaparin were performed for higher-risk patient subgroups, including variables such as age, smoking, obesity, cardiovascular diseases, type of anesthesia, and concomitant medications. Adverse events studied included serious bleeding episodes, wound dehiscence, wound hematoma, and infections.
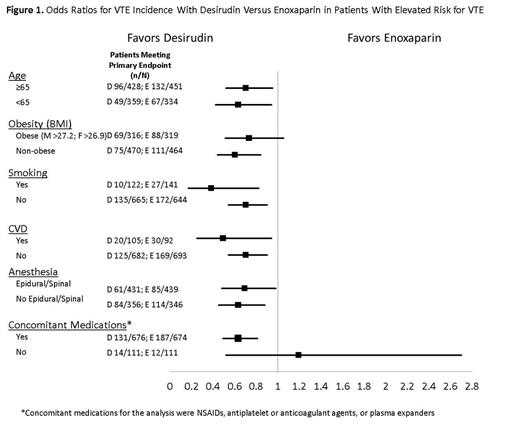
RESULTS. Post hoc analyses for intent-to-treat patients evaluable for the primary outcome (desirudin n=802; enoxaparin n=785) reveal favorable odds ratios (OR) for desirudin versus enoxaparin in VTE incidence (Figure 1) across a range of high-risk patient subgroups, including (1) patients aged 65 or older (OR 0.70); (2) obese patients (BMI >27.2 kg/m2 for males and >26.9 kg/m2 for females) (OR 0.73); (3) smokers (OR 0.38); (4) patients with cardiovascular disease (OR 0.49); (5) patients who had epidural or spinal anesthesia (OR 0.69); and (6) patients who received concomitant medications such as NSAIDs, antiplatelet or anticoagulant agents, or plasma expanders (OR 0.63). In addition, risk factor analysis by logistic regression showed that factors associated with a statistically significant increase in the probability of adverse events included: age 65 or over (OR 1.93, P=.0001); history of venous thromboembolic disease (OR 1.80, P=.02); and obesity (OR 1.46, P=.0046). Post hoc analyses of hemorrhagic events revealed no statistically significant difference between patients who received desirudin and those who received enoxaparin (desirudin 336/1042, enoxaparin 341/1036; P=.779).
CONCLUSION. Favorable ORs for VTE incidence with desirudin compared with enoxaparin were found for patients ≥65 years of age, who were obese, who smoked, had cardiovascular disease, received epidural or spinal anesthesia, or were receiving various concomitant medications. Eriksson’s previous report noted a significantly lower rate of proximal DVT (4.5% versus 7.5%, P=.01) and a lower overall rate of DVT (18.4% versus 25.5%, P=.001) for desirudin compared with enoxaparin. The efficacy results reported here in higher-risk patients, together with the finding that desirudin has a hemorrhagic event profile similar to that of enoxaparin, suggest that desirudin may be a preferred agent over enoxaparin for thromboprophylaxis in higher-risk patients undergoing THR.
Levy:Marathon Pharmaceuticals, LLC: Consultancy. Nutescu:Marathon Pharmaceuticals, LLC: Consultancy. Meyer:Marathon Pharma: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal