Abstract
Introduction: Factor XI (FXI) is a homodimeric serine protease enzyme, which is produced in the liver and circulates in the plasma complexed with high molecular weight kininogen. Approximately 1% of FXI is presented in platelets. After the activation of the extrinsic pathway of coagulation, thrombin activates FXI, and this reaction has an important role in the amplification of coagulation reaction. Congenital FXI deficiency is characterized by decreased levels or activity of FXI in the plasma, and may cause an inherited bleeding disorder. Congenital FXI deficiency is very rare (less than 1/100.000) in the general population. The factor XI gene is located on the long arm of chromosome 4 (4q34-35), consists of 15 exons and 14 introns, and encodes a 607 amino acid protein. FXIa has two chains: the light chain contains the catalytic site whereas the heavy chain contains the four tandem repeat sequences (Apple domains, A1 to A4). Mutations of the FXI gene (F11 gene) leading to FXI deficiency can occur in the Apple domains or the catalytic site. One of the most common mutations is a missense mutation in the Apple 4 domain, which is important for FXI dimerization, resulting in low serum levels of FXI. Some mutations can cause a moderate to severe FXI deficiency by producing aberrant proteins. Here we present a consanguineous family with FXI deficiency.
Patients and methods: In this study a family with FXI deficiency was evaluated. The index patient was a 10 years old boy with bleeding diathesis (excessive bleeding after tooth extraction). His aPTT level was 84,3 seconds (N: 32-39 seconds), FXI activity was 0%, and he was diagnosed with FXI deficiency in 2010. His parents were related. The father had a mild bleeding tendency with prolonged aPTT (48.2 seconds). FXI activity was found to be 4%. The mother and second child had no bleeding history with mildly decreased FXI activities (40% and 60%, respectively). We performed a mutational analysis for whole family, including his grandparents. Genomic DNA was extracted from whole blood. All exons and approximately 30bp exon-intron boundaries of F11 gene were amplified using sets of designed primers. PCR reactions were performed using 2X PCR master mix (HibriGen Biotech R&D, Istanbul, Turkey), primers and 25-30 ng genomic DNA. Amplified fragments were sequenced.
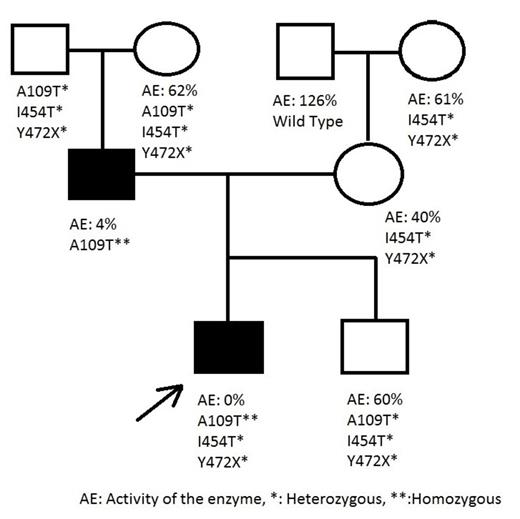
Results: We obtained the results shown in Figure 1.
The patient and his father had a A109T homozygous mutation for F11; the index patient also had heterozygous I454T and Y472X mutations. The presence of a homozygous A109T mutation in father and index patient caused severe FXI deficiency. The mother and second child had heterozygous I454T and Y472X mutations. As shown in the pedigree, I454T and Y472X compound heterozygosity moderately decreases the activity of FXI.
Conclusions: Approximately 220 F11 mutations have been reported to date, affecting both the catalytic and Apple domains. In this family, we found two novel mutations, I454T and Y472X, associated with a homozygous A109T mutation. This is the first case reported with a homozygous A109T mutation in the literature. Previously Guella et al. reported a heterozygous A109T mutation in an Italian family with FXI deficiency (Thromb Haemost, 2008). They showed that exon-skipping had occured due to a heterozygous A109T mutation. They explained that the unchanged enzyme activity was due to a non-sense mediated RNA decay mechanism. Our cases confirmed their results, such that a heterozygous A109T mutation did not affect enzyme activity; the enzyme activity of a person who has two heterozygous mutations (Y472X and I454T) is the same as who has three heterozygous mutations (A109T, Y472X and I454T). However, when the A109T was homozygous like in our patient and his father, the enzyme activity decreased by approximately 96% as shown in the pedigree. Another interesting point was the presence of a homozygous A109T mutation in the patient while the mother had no A109T mutation. This can be explained by somatic recombination as seen in variable diversity junction (VDJ) recombination in the immune system. Further expression studies evaluating the effects of these mutations will improve our understanding of the functional and structural features of the FXI enzyme.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal