Abstract
Background
Hemophilia A is an X-linked inherited bleeding disorder caused by a deficiency of clotting factor VIII (FVIII). It is treated by infusion of FVIII clotting factor concentrate with dosing based primarily on bodyweight. Previous studies evaluating perioperative dosing strategies in hemophilia conclude that improvement with regard to consumption of clotting factor concentrates is possible. However, the magnitude and complexity of the problem has not yet been addressed. Moreover, guidelines to optimize treatment have been lacking.
Methods
In a retrospective multicenter study, we evaluated perioperative management in hemophilia A patients with clotting factor VIII (FVIII) plasma levels below 0.05 IUml-1 by quantification of perioperative infusion of clotting factor concentrate and achieved FVIII plasma levels, while exploring possible modifiers of clotting factor concentrate consumption.
Results
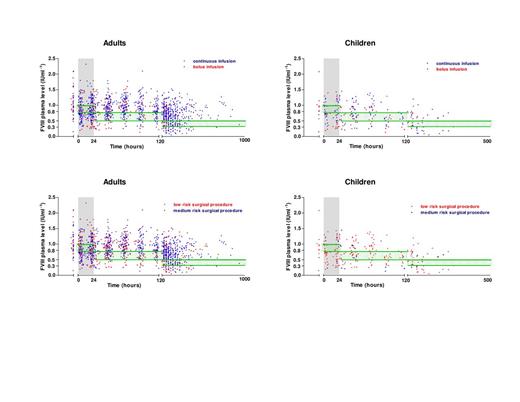
Data was collected in a total of 198 surgical procedures in 119 patients; 75 adults (140 surgical procedures; median age: 48 years; median weight: 80 kg) and 44 children (58 surgical procedures; median age: 4 years; median weight: 19 kg). In adults, mainly medium risk surgical procedures (n=86; 61%) were performed, which were most often orthopedic procedures (n=91; 65%). Children mainly underwent low risk surgical procedures (n=47; 81%), most frequently an insertion or removal of a central venous device (n=31; 53%). The median duration of hospitalization in adults and children was respectively nine (IQR 5-14) and seven (IQR 6-10) days; p=0.09. The median amount of clotting factor concentrate infused per surgical procedure was 26,100 IU (69 IUkg-1day-1). Depending on post-operative day, 67-81% of achieved FVIII plasma levels were outside of the predefined target range recommended by National Hemophilia Consensus. Moreover, 45% of FVIII plasma levels were below target range between 0-24 hours after surgery with the median deviation below the lowest required target level of 0.17 IUml-1. More than six days after surgery, 75% of the FVIII plasma levels were above target range with the median deviation above the highest target level of 0.31 IUml-1. No significant difference in frequency of under dosing or overdosing was demonstrated in adults or children. Neither was mode of administration of replacement therapy (continuous or bolus), or type of surgical procedure (low or medium risk), significantly related with under dosing or overdosing (Figure 1A-D). Moreover, under dosing was not correlated with clinical bleeding and overdosing did not lead to observed cases of vascular thrombosis. Overall, in this study population the total amount of clotting factor concentrate under dosed amounted to 422,000 IU and overdosed amounted to 3,320,300 IU, when calculated using the median deviation of achieved FVIII levels in comparison to the predefined target range and an in vivo recovery of 2.0 IUml-1 per 1 IUkg-1, a crude median half-life of FVIII concentrate of 12 hours and an overall median hospitalization period of nine days. Importantly, a reduction of clotting factor concentrate consumption of approximately 49% would have been realized if predefined plasma target levels could have been achieved.
Conclusion
Targeting of clotting FVIII levels in the perioperative setting is complex and forms a “moving target” for treating professionals. Optimization of dosing strategies by construction of algorithms with minimization of both under dosing and overdosing is obligatory to improve quality of care with a reduction of bleeding risk, a possible decrease of clotting factor concentrate consumption and potential cost reduction of treatment.
Figure 1: Achieved FVIII plasma levels in adults (1A and 1C) and children (1B and 1D) receiving FVIII clotting factor replacement therapy. Figure 1A and 1B: Achieved FVIII plasma levels of patients treated by continuous infusion (blue dots) and by bolus infusions (red dots). Figure 1C and 1D: Achieved FVIII plasma levels of patients treated for a medium risk surgical procedure (blue dots) and patients treated for a low risk surgical procedure (red dots). Predefined target levels (green line) as stated by the Dutch Hemophilia Consensus are depicted as green boxes (Leebeek et al. 2009)
Figure 1: Achieved FVIII plasma levels in adults (1A and 1C) and children (1B and 1D) receiving FVIII clotting factor replacement therapy. Figure 1A and 1B: Achieved FVIII plasma levels of patients treated by continuous infusion (blue dots) and by bolus infusions (red dots). Figure 1C and 1D: Achieved FVIII plasma levels of patients treated for a medium risk surgical procedure (blue dots) and patients treated for a low risk surgical procedure (red dots). Predefined target levels (green line) as stated by the Dutch Hemophilia Consensus are depicted as green boxes (Leebeek et al. 2009)
Lock:ZonMW: Research Funding; Baxter: Research Funding. Hazendonk:ZonMW: Research Funding; Baxter: Research Funding. Meijer:Bayer Schering Pharma: Research Funding, speakers fee, travel support, outside the submitted work Other; Sanquin: Research Funding, speakers fee, outside the submitted work, speakers fee, outside the submitted work Other; Boehringer Ingelheim: speakers fee, outside the submitted work, speakers fee, outside the submitted work Other; Baxter: Research Funding, travel support, outside the submitted work, travel support, outside the submitted work Other. Driessens:Baxter: unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work Other; Bayer Schering Pharma: unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work, unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work Other; CSL Behring: unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work, unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work Other; Eurocept: unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work, unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work Other; Novo Nordisk: unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work Other; Pfizer: unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work Other; Sanquin: unrestricted grant for meetings and educational courses with hemophilia patients and members of the Netherlands Hemophilia Patient Society, outside the submitted work Other. Fijnvandraat:Baxter: European Hemophilia Treatment and Standardisation Board sponsored by Baxter Other; CSL Behring: Research Funding; Pfizer: has given lectures at educational symposiums organized by Pfizer, outside the submitted work, has given lectures at educational symposiums organized by Pfizer, outside the submitted work Other, Research Funding; Bayer Schering Pharma: has given lectures at educational symposiums organized by Bayer, outside the submitted work, has given lectures at educational symposiums organized by Bayer, outside the submitted work Other. Leebeek:CSL Behring: has served on advisory boards of CSL Behring, outside the submitted work Other, Research Funding; Baxter: has served on advisory boards of Baxter, outside the submitted work, has served on advisory boards of Baxter, outside the submitted work Other. Cnossen:Novo Nordisk: Educational funding Other, Research Funding; Bayer Schering Pharma: Educational funding and travel support, Educational funding and travel support Other, Research Funding; Baxter: Research Funding, Travel support, Travel support Other; Pfizer: Educational funding and travel support, Educational funding and travel support Other, Research Funding; ZonMW: Research Funding; Novartis: Educational funding and travel support Other, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal