Abstract
Background: It is known that antiplatelet therapy has become an important treatment of coronary artery disease. However, the standardized monitoring methods of antiplatelet drugs remain unestablished, since platelets are very easy to be activated, and the platelet function testing in vitro is difficult to standardization. Large-scale clinical trials can only rely on the probability of occurrence of ischemic time and massive bleeding in patients to determine the appropriate dose and type of antiplatelet drugs. Therefore, we hope to assess platelet function before and after antiplatelet therapy by using a variety of testing methods which include the platelet aggregation, thrombelastography, platelet VASP and CD62P expression.
Objectives: In this study, we sought to optimize the combination of these methods and explore an effective, simple method for the monitoring of antiplatelet treatment.
Methods: A total of 309 inpatients that were suffered from suspected coronary artery disease, and underwent CAG+PCI were enrolled between June 2013 and January 2014 in Department of Cardiology of Sun Yat-sen Memorial Hospital. All the eligible patients were divided into clopidogrel group (75 mg/d)£¬ aspirin group (100mg/d) and aspirin plus clopidogrel group (dual antiplatelet therapy, aspirin 100 mg/d plus clopidogrel 75 mg/d), and those with no previous history of antiplatelet treatment as the healthy control group. The testing indicators of platelet function were platelet aggregation, platelet CD62P expression and vasodilator stimulated phosphoprotein (VASP) phosphorylation level (PRI). These indicators were detected in clopidogrel and aspirin groups in 1 month and 3 months after antiplatelet therapy respectively, and detected separately in 2 hours, 1 month, and 3 months after coronary stent implantation in aspirin plus clopidogrel group. Platelet aggregation was measured by turbidimetric test with ADP and arachidonic acid (AA) as inducers. Correlation analysis of each indicator was also carried out.
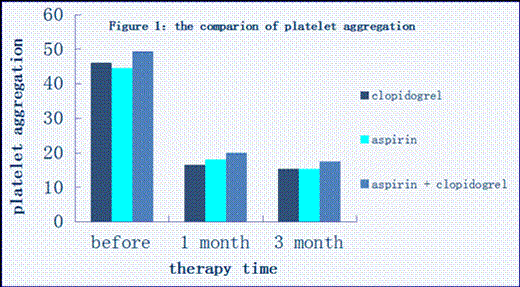
Results: The platelet aggregation, in 1 and 3 months after antiplatelet therapy in clopidogrel group, aspirin group, clopidogrel plus aspirin group, was significantly lower than that before treatment (Figure 1, P <0.05). Especially in clopidogrel plus aspirin group, in 2 hours after surgery, there was an obvious decrease and it decreased more in 3 months than 1 month. Platelet CD62P expression, in 3 months after antiplatelet therapy in the three groups, showed a significant decrease when compared with that before treatment (Figure 2, P<0.05), and CD62P expression was the lowest in clopidogrel plus aspirin group than monotherapy groups. PRI levels in clopidogrel, clopidogrel plus aspirin group were significantly decreased than that before treatment (Figure 3, P<0.05), but no difference was found in aspirin group. There was positive correlation between platelet aggregation and platelet CD62P expression between aspirin group (P< 0.05, r=0.64302) and clopidogrel plus aspirin group (Figure 4, P<0.05, r=0.43632). However, no correlation between platelet aggregation rate and PRI was observed in 3 groups after treatment.
Conclusions: This study not only demonstrates that either monotherapy or combination therapy can reduce platelet aggregation rate, platelet CD62P expression and PRI levels, but also shows that platelet aggregation and CD62P expression have good consistency and it can be more meaningful to detect antiplatelet therapy together. In our study, we also detected 300 cases of diabetes and found that CD62P expression decreased significantly after antiplatelet therapy indicating that it could be used as the specific indicator to monitor platelet function. It is noteworthy that PRI is more sensitive as the monitoring index of clopidogrel. But our results do not support the good consistency between platelet aggregation rate and PRI levels. The small number of cases may limit the generalizability results. Additional research is needed to increase the sample size to require validation and continue to monitor the indicators of a year to two years after, as patients in our study are still being monitored.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal