Abstract
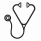
Introduction: Atypical hemolytic uremic syndrome (aHUS) is a progressive, life-threatening disease of uncontrolled and chronic complement activation, leading to systemic thrombotic microangiopathy (TMA) and severe end-organ damage. Prior to an effective pharmacologic treatment, and despite intensive management with plasma exchange/plasma infusion, up to 25% of children died during the initial presentation, and up to 48% reached end-stage renal disease or died 5 years after disease onset. In a prospective study of children and adolescents with aHUS, eculizumab (ECU), a terminal complement inhibitor, was shown to inhibit TMA and improve hematologic outcomes and renal function by 26 weeks. Here, we report 1-year data from this prospective trial.
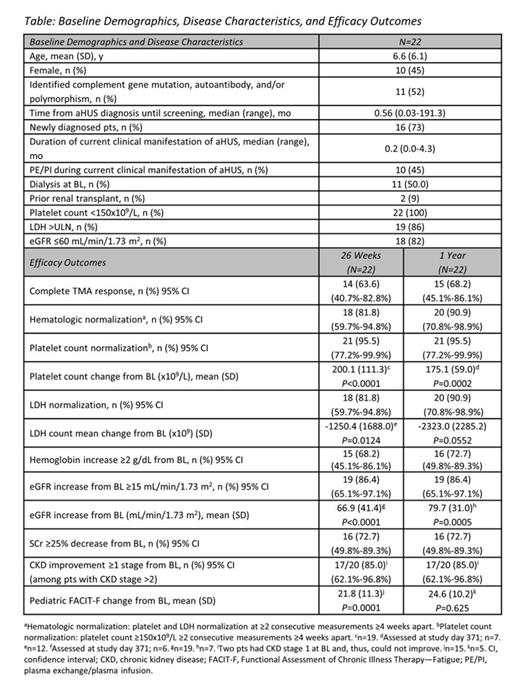
Methods: This was an open-label, single-arm, Phase 2 trial of ECU in pediatric patients (pts) with aHUS. Inclusion criteria included platelet count less than the lower limit of normal (LLN) at screening and at baseline (BL), lactate dehydrogenase (LDH) ≥1.5 times the upper limit of normal (ULN) at the start of the current manifestation, and elevated serum creatinine (SCr) at screening. An identified complement abnormality was not required. Pts with STEC-HUS (Shiga toxin + E. coli) or severe ADAMTS13 deficiency (<5%) were excluded. The primary endpoint was the proportion of pts who achieved complete TMA response, defined as hematologic normalization (platelet count ≥150x109/L; LDH ≤ULN), and improvement of renal function (≥25% decrease in SCr from BL, confirmed by ≥2 consecutive measurements obtained ≥4 weeks apart). Dosing was based on weight cohorts, and the regimen was designed with investigators and regulatory agencies to ensure that ≥95% of pts had complete and sustained terminal complement inhibition (defined as >80% inhibition in a hemolytic assay), including in times of increased complement activity (eg, infection or surgery).
Results: 22 pts (aged 1 month to 17 years) were enrolled and 19 completed 26 weeks of treatment. The median time from the current manifestation to enrollment was 0.20 months (range, 0.03–4.26). At the 1-year update, the mean (SD) treatment duration was 12.5 (6.38) months, with a median (range) of 12.6 (0.0–24.5) months. At week 26, 14 pts (64%) achieved the primary endpoint of complete TMA response (Table), and that number increased to 15 (68%) at 1 year. Platelet levels (Fig 1) and estimated glomerular filtration rate (eGFR) (Fig 2) increased significantly from BL through the 26-week study period, and those gains were maintained or further improved at 1 year. Nine of 11 pts (82%) on dialysis at BL discontinued dialysis, and all remained dialysis-free at 1 year. 11 pts not on dialysis at BL also remained dialysis-free at 1 year. Quality of life significantly improved. ECU was well tolerated and there were no meningococcal infections or deaths.
Conclusions: Longer-term analysis at 1 year further demonstrates the safety and efficacy of ongoing ECU therapy in pediatric aHUS pts. It is interesting to note that renal function, as represented by eGFR, further increased between weeks 26 and 1 year: this highlights the need to pursue ECU treatment over the long term. These findings show that treatment with ECU results in life-altering outcomes, in stark contrast to the natural history of the disease.
Greenbaum:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ardissino:Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Al-Akash:Alexion Pharmaceuticals: Honoraria, Speakers Bureau. Lieberman:Alexion Pharmaceuticals: Honoraria, Speakers Bureau; Questcor: Honoraria, Speakers Bureau. Rees:Alexion Pharmaceuticals: Honoraria. van de Kar:Alexion Pharmaceuticals: Member of International Advisory Board of aHUS Other. Vande Walle:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Ogawa:Alexion Pharmaceuticals: Employment. Bedrosian:Alexion Pharmceuticals: Employment. Licht:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Achillon Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
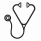
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal