Abstract
Introduction:Neutropenia is a common adverse effect of myelosuppressive chemotherapy and may increase the risk of infection for cancer patients. However there is limited understanding about the quantitative relationships between severity and duration of chemotherapy induced neutropenia (CIN) and the risk of infection among patients undergoing chemotherapy to treat non-myeloid malignancies.
Methods: The current study is a later analysis of data from six Amgen sponsored clinical trials. We combined individual data from adult patients with non-myeloid cancer who received no granulocyte colony-stimulating factor (G-CSF) during the first cycle of myelosuppressive chemotherapy and had absolute neutrophil count (ANC) measured at least 3 times per week. The primary endpoint is occurrence of an infection related hospitalization (identified from a review of hospitalization records). The secondary endpoint is occurrence of fever (≥38.2°C) or an infection related hospitalization in combination with different grades of CIN (grade 4: ANC <0.5 x 109/L or grade 3/4: ANC<1.0 x 109/L) on the same day, -1 day, or + 2 days. Descriptive analyses were conducted to characterize the ANC nadir and time to ANC nadir. An additional variable was created to measure both the duration and severity of CIN: area over the curve (AOC) of ANC, which was calculated as the area below the threshold of 0.5 x 109/L (or 1.0 x 109/L) and above ANC-time response curve in the first chemotherapy cycle. Time-dependent Cox proportional hazards models were used to quantify the hazard of first infection associated with duration of grade 4 or grade 3/4 CIN as well as the hazard associated with AOC, all in the first chemotherapy cycle.
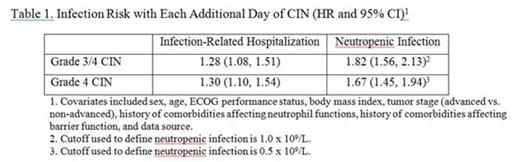
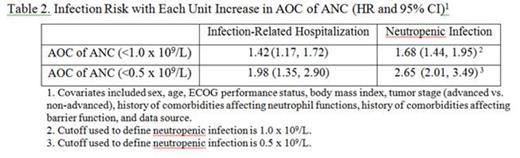
Results: Our study analyzed data from 271 patients who had one of four cancer types: small cell lung cancer (56.1%), non-Hodgkins lymphoma (24.4%), oral cavity and pharyngeal cancer (11.4%), and breast cancer (8.1%). About 63.8% of the patients had advanced cancer and 77.5% of them received chemotherapy regimens with a high risk of developing febrile neutropenia. The ANC trajectory is shown in the figure below. The median of daily ANC reached its minimum at day 12 with a value of 0.2 x 109/L. In the first chemotherapy cycle, 18.8% had infection-related hospitalizations; 42.8% and 41.0% of patients developed grade 3/4 and grade 4 neutropenic infections, respectively. For each additional day that patients had grade 3/4 and grade 4 CIN was associated with a 28% and 30% increase in the risk of infection related hospitalization, respectively (Table 1). Each unit (day×109/L ANC) increase of AOC (below 0.5 x 109/L) was also associated with a significantly increased risk of infection related hospitalization (HR: 1.98; 95% CI: 1.35-2.90) (Table 2).
Conclusions: Risk of infection increases dramatically with each additional day of grade 3 or 4 CIN. Interventions that limit the duration and extent of CIN are of critical importance to preventing infection among cancer patients receiving chemotherapy.
Figure. ANC Trajectory (Median and Interquartile Range) in the First Chemotherapy Cycle
Li:Amgen: Employment, Equity Ownership. Shih:Amgen: Consultancy. Klippel:Amgen: Employment, Equity Ownership. Reiner:Amgen: Employment, Equity Ownership. Page:Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal