Abstract
BACKGROUND: DS3 scoring is a method of expressing an integrated assessment of disease burden in a given patient based on bone, hematologic and visceral domains. A DS3 severity score index for adult patients with type 1 Gaucher disease (GD1) was developed and tested for validity, reliability and feasibility (Weinreb NJ et al. Genet Med 2009; 12:1-8). However, the DS3 score has not yet been applied to large numbers of patients across different clinics. We developed a consortium of five geographically separated North American GD treatment centers to further investigate the DS3 score as a tool for initial assessment, long term follow up and evaluation of treatment responses.
METHODS: 133 adult patients with a history of IV enzyme treatment (ERT) for GD1 were recruited under informed consent. All patients agreed to allow International Collaborative Gaucher Registry (ICGG, supported by Genzyme) data with additional clinical phenotyping to be used to calculate DS3 and annotate longitudinal changes. An ICGG independent data center collated the information. Baseline DS3 scores were calculated around the date of initial treatment. Follow up scores were calculated annually with a ±3 month window. Because the calculation of DS3 scores is sensitive to missing data, some values were imputed.
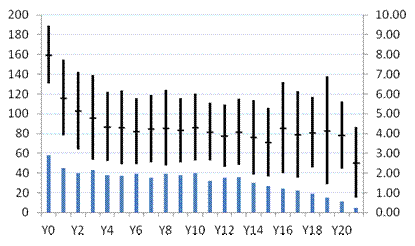
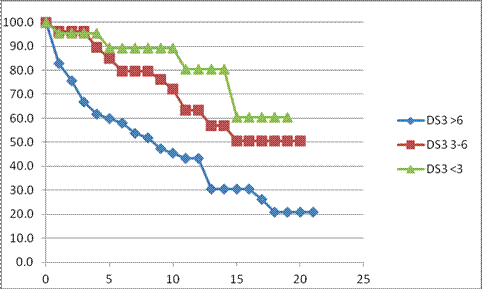
RESULTS: Patient characteristics: 68 patients (51%) identified all grandparents as Ashkenazi Jews. 48 of 52 N370S/N370S patients had complete or partial Ashkenazi Jewish ethnicity. N370S/L444P was the most common genotype among non-Jewish patients. 118 patients had at least one N370S allele. Median age at the time of GD1 diagnosis was 28 (0-85) y. 38 patients (26.6%) had a history of splenectomy. Treatment commenced at a median age of 45 (6-87) y with a median follow up on treatment of 14 (1-23) y. Baseline DS3 scores: In 58 patients, pre-treatment scores 6.0-14.9 (median 7.84) signified marked disease severity. 31 patients (53%) had a history of pre-treatment splenectomy. Median age at diagnosis was 18 (0-68) y. Median age at first treatment was 44 (7-69) y. Only 11 patients (19%) were N370S homozygous. 44 patients (76%) had major bone pathology (avascular necrosis, fractures, lytic lesions) pre-treatment. Fifty-three patients had moderate disease severity (DS3 3.00-5.93; median 4.33). Splenectomy prevalence was 15.1%. Median age at diagnosis was 32 (4-85) y; median age at first treatment was 50 (6-87) y. 26 patients (49%) were N370S/N370S. 9 patients (17%) had major pre-treatment bone disease. Twenty-two patients had mild severity disease (DS3 0.40-2.93; median 2.40). Median age at diagnosis was 40 (1-71) y; median age at first treatment was 44 (9-73) y. None had splenectomy or major pre-treatment bone disease. 15 patients (68%) were N370s/N370S. Response to treatment: Health-state transitions occurred primarily during the first 5 treatment years. Of 58 patients with marked pre-treatment disease, 33% remained so after Y1. At Y5, 14% were “marked,” 50% “moderate,” 19% “mild,“ 17% not evaluable (NE). Of 53 “moderate” patients, 38% continued so after Y1. At Y5, 30% were “moderate,” 49% “mild,” 21% NE. No patient worsened in severity category. Of 22 “mild” patients, all remained so at Y1, At Y5, 2 patients had transitioned to “moderate” (DS3 scores 3.07 and 3.17); 64% remained “mild” and 27% were NE. Among evaluable marked severity patients, 76% had clinically important improvement (defined as a -3.1 ΔDS3 from baseline) at Y5. In “moderate” patients with initial DS3 scores 4.58-5.93, 41% had clinically important improvement at Y5. No patients with baseline DS3 scores <4.50 had a clinically important response.
CONCLUSIONS: DS3 is an effective tool for assessing pre-treatment disease burden in GD1 and for monitoring response to therapy. It is consistent with indirect indicators of GD1severity such as genotype and early age of diagnosis and symptom onset. ERT is associated with clinically important improvement in DS3 scores in patients with high severity scores. Nevertheless, even with sustained improvement in DS3 scores, GD1 patients on ERT, especially those with risk factors such as splenectomy and pre-treatment bone pathology are at continual risk for emerging major bone complications (Fig 1,2). SUPPORT: Independent Investigator Grant from Genzyme.
DS3 score
“Marked” severity patients: change in mean (+/-) SD DS3 scores with ERT
Years
Weinreb:Genzyme, a Sanofi Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Shire HGT: Honoraria, Speakers Bureau; Pfizer Corporation: Consultancy, Honoraria, Speakers Bureau. Shankar:Genzyme, a Sanofi Company: Consultancy, Honoraria, Travel reimbursement, received funds for fellowship training, for educational and patient meetings Other; Shire: Consultancy, Honoraria, Travel reimbursement, received funds for fellowship training, for educational and patient meetings, Travel reimbursement, received funds for fellowship training, for educational and patient meetings Other; Protalix-Pfizer: Consultancy, Honoraria, Travel reimbursement, received funds for fellowship training, for educational and patient meetings, Travel reimbursement, received funds for fellowship training, for educational and patient meetings Other; Biomarin: Consultancy, received funds for fellowship training, for educational and patient meetings, received funds for fellowship training, for educational and patient meetings Other; Actelion: Consultancy, received funds for fellowship training, for educational and patient meetings Other. Rosenbloom:Genzyme, a Sanofi Company: Consultancy, Honoraria, Travel reimbursement Other. Finegold:Genzyme, a Sanofi Company: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shire HGT: Honoraria, Membership on an entity's Board of Directors or advisory committees; Protalix Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal