Abstract
Background: Iron overload is a leading cause of morbidity and mortality in beta thalassemia and chelation therapy remains the mainstay in reducing iron burden. For effective chelation, optimal drug dosage with close monitoring of side effects is crucial. The safety and efficacy of deferasirox has been extensively studied in adult population. We aimed at evaluating the liver enzyme changes and safety profile of deferasirox in pediatric patients with thalassemia major.
Materials and Methods: A retrospective study of 30 patients (range 2-15 years) with thalassemia major on regular follow up at the Pediatric Day care Center, Sultan Qaboos University Hospital was performed to evaluate the side effects of deferasirox over a mean of 15 month follow-up period. Data from electronic patients' records was collected for age, gender, serum ferritin, alanine transaminase, (ALT), aspartate transaminase (AST), dose and side effects of deferasirox. Data were analyzed using SPSS software version 19.
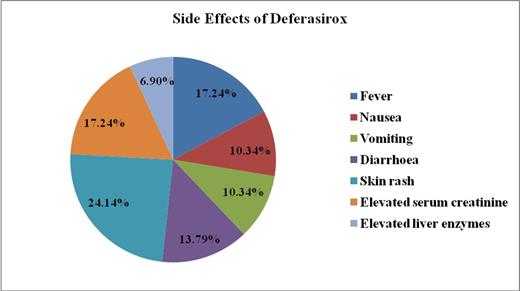
Results: Thirteen (44.8 %) patients had either ALT or AST elevation above 2 times upper limit of normal (ULN). Except for two (6.8%) patients with enzyme elevations more than 5 times ULN, the majority of patients had mild transaminitis. None of the patients had liver enzyme elevation above 10 times ULN. The other side effects included fever (17.24%), nausea and vomiting (10.34% each), diarrhoea (13.79%), skin rash (21.4%), elevated serum creatinine with either 2 consecutive readings more than 33% of baseline level and/or single reading above 60% baseline (17.24%). The mean serum ferritin dropped from initial baseline level of 1236.21 ± 354 ng/ml (Range 534 -2821 ng/ml) to a level of 950 ± 320 ng/ml (Range 550-1900 ng/ml) at the end of the study period; with a mean deferasirox dose of 32.72 ± 4.79 mg/kg/day.
Conclusion: Majority of our patients had mild transaminitis not requiring dose modification or interruption of chelation. Patients with significant elevations of liver enzymes more than 5 times ULN showed prompt recovery of transaminitis within 4-5 weeks of dose reduction of deferasirox by 5 mg/kg/day and future dose increments were well tolerated. Except for significant elevations in serum creatinine requiring dose reduction or short interruption, the other adverse events were well tolerated and did not warrant dose modification. Deferasirox was effective in reducing serum ferritin levels and was well tolerated in our young patients with thalassemia major.
| PatientCharacteristics . | |
|---|---|
| Numberof patients, n . | 30 . |
| Meanage of patients, years Range,years | 7.24± 3.4 2-15 |
| Meanserum ferritin, ng/ml Range,ng/ml | 1236.21± 354 534-2821 |
| Meandose of deferasirox (mg/kg/day) | 32.72± 4.79 |
| PatientCharacteristics . | |
|---|---|
| Numberof patients, n . | 30 . |
| Meanage of patients, years Range,years | 7.24± 3.4 2-15 |
| Meanserum ferritin, ng/ml Range,ng/ml | 1236.21± 354 534-2821 |
| Meandose of deferasirox (mg/kg/day) | 32.72± 4.79 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal