Abstract
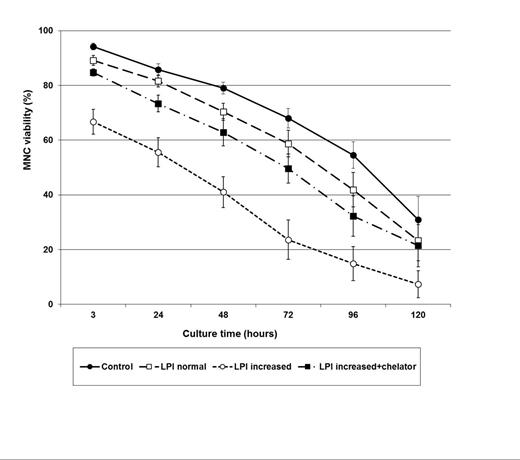
Myeloablative conditioning for hematopoietic stem cell transplantation (HSCT) leads to a fast increase of non-transferrin bound iron, including the labile plasma iron (LPI) pool, mainly due to suppression of erythropoiesis. It has been suggested that increased LPI, the redox-active and toxic form of iron, may cause cell damage and ultimately lead to tissue toxicity and other complications commonly observed in the early post-HSCT period. However, this assumption has not been reported yet in this setting. In order to evaluate if increased LPI levels following conditioning can cause cell toxicity, we assessed its impact on viability of mononuclear cells obtained from eight patients undergoing autologous HSCT for multiple myeloma (n=4) and lymphoma (n=4). Firstly, LPI was measured in plasma samples obtained from each patient before (baseline) and after conditioning (on day 0), revealing normal levels (<0.5µM) in all baseline samples (mean: 0.2µM; range: 0-0.4µM) and increased levels in all day 0 samples (mean: 2.0µM; range: 1,1-4.0µM). Then, on day 0, an aliquot from peripheral blood stem cells collected by apheresis was obtained before infusion and mononuclear cells were isolated by Ficoll gradient and cultured in DMEM culture media in duplicates for five days in four experiments: i) without addition of plasma (control), ii) with addition of autologous plasma containing normal LPI levels (baseline sample), iii) with addition of autologous plasma containing increased LPI levels (day 0 sample) and iv) with autologous plasma containing increased LPI levels (day 0 sample) in addition of an iron chelator (50mM deferiprone). Mononuclear cells were cultured with autologous plasma on a 1:1 volume ratio. Viability of cultured mononuclear cells was determined by trypan blue method at 3, 24, 48, 72, 96 and 120 hours. Cell viability decreased over time in all experiments (p < 0.001; Figure). There was no difference in cell viability between cells cultured without addition of plasma (control) and cells cultured with plasma containing normal LPI levels. Cells cultured with plasma containing increased LPI levels presented the lowest viability in relation to the other three culture experiments at all time points (p<0,001). The viability of cells cultured with plasma containing increased LPI levels in addition of iron chelator was similar to that of cells cultured with normal LPI levels, also at all time points (p>0,05). In conclusion, these results indicate that increased LPI levels can decrease substantially the viability of mononuclear cells, an effect that can be prevented or at least attenuated by the use of iron chelators.
Changes on viability of mononuclear cells (MNC) cultured without and with autologous plasma containing normal LPI levels, increased LPI levels and increased LPI levels in addition of the iron chelator (deferiprone). (n=8 patients; Values represent mean±SEM).
Changes on viability of mononuclear cells (MNC) cultured without and with autologous plasma containing normal LPI levels, increased LPI levels and increased LPI levels in addition of the iron chelator (deferiprone). (n=8 patients; Values represent mean±SEM).
Naoum:Novartis Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal