Abstract
Introduction: Total hip replacement (THR) surgery is effective in returning patients to function and improves health-related quality of life. However, venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a frequent complication of THR surgery that can be life threatening. Anticoagulation with low molecular weight heparin (LMWH) has been shown to be efficacious for lowering the risk of VTE among patients undergoing THR surgery. However, LMWH has biological limitations, such as the inability to inhibit clot-bound thrombin. Desirudin is a highly potent, selective, irreversible inhibitor of thrombin and inactivates both free and clot-bound thrombin. In the phase III, randomized, double-blind, clinical trial that compared the efficacy and safety of desirudin to LMWH among patients undergoing THR surgery, desirudin significantly reduced the overall rate of VTE relative to LMWH. Based on differences in event rates in the trial, this study estimated and compared the medical costs of THR surgery patients treated with desirudin vs. patients treated with LMWH for VTE prophylaxis.
Methods: Event rates of efficacy and safety endpoints, including VTE defined as the overall rate of DVT, PE, or death related to thromboembolism, major bleeding (MB), clinically relevant non-major bleeding (CRNMB), and other minor bleeding, were obtained from published trial data. Incremental annual medical costs among patients with clinical events from a U.S. payer perspective were obtained from published literature. Costs were inflation adjusted to 2013 levels. Differences in total annual medical costs associated with clinical endpoints for desirudin vs. LMWH were then estimated. One-way and Monte Carlo multivariable sensitivity analyses were additionally carried out.
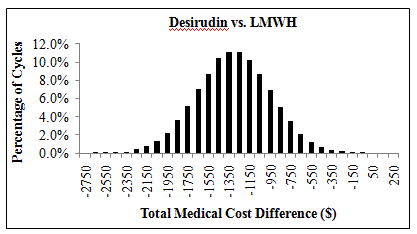
Results: Desirudin treatment was associated with a 6.9% lower rate of VTE among patients undergoing THR surgery. As a result of the reduction in VTE event rate, annual medical costs were reduced by $1,250 per patient treated with desirudin in comparison to that of a patient treated with LMWH (Table). Univariate (one-way) sensitivity analysis showed that rates of VTE and MB had the greatest impact on the medical cost differences between desirudin and LMWH. The results of the 10,000 cycles of Monte Carlo multivariable analysis showed that the medical cost offset of desirudin vs. LMWH had a 95% interval range of -$562 to -$1,955, with 100% of the cycles showing a cost reduction <$0 (Figure).
Conclusions: According to the estimate from this economic model, among patients undergoing THR surgery, VTE prophylaxis with desirudin is associated with reduced medical costs resulting from better efficacy in preventing VTE than LMWH. The efficacy and safety profile of desirudin and the estimated cost offset compared to LMWH make desirudin a good option for VTE prophylaxis among THR surgery patients in the U.S. Further evaluation may be needed to validate the economic model results in the real-world setting.
Estimated Medical Cost Differences Among Patients who Undergo THR Surgery Treated with Desirudin vs. LMWH
| Outcome . | Desirudin vs. LMWH ($/patient-year) . |
|---|---|
| Venous Thromboembolism | -$1,299 |
| Major Bleedings | $52 |
| Clinically Relevant Non-major Bleedings | $0 |
| Other Minor Bleedings | -$4 |
| Total Medical Cost | -$1,250 |
| Outcome . | Desirudin vs. LMWH ($/patient-year) . |
|---|---|
| Venous Thromboembolism | -$1,299 |
| Major Bleedings | $52 |
| Clinically Relevant Non-major Bleedings | $0 |
| Other Minor Bleedings | -$4 |
| Total Medical Cost | -$1,250 |
Negative numbers indicate a cost reduction vs. LMWH
Distribution of Total Medical Cost Differences from 10,000 Cycles of Monte Carlo Simulation: Desirudin vs. LMWH
Deitelzweig:3-D Medical Services, Marathon Pharma: Consultancy, Research Funding. Nutescu:Marathon Pharma, Janssen, Anticoagulation Forum, American College of Clinical Pharmacy, National Blood Clot Alliance, NHLBI: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Meyer:Marathon Pharma: Employment. Lin:Marathon Pharma: Consultancy, Research Funding. Lingohr-Smith:Marathon Pharma: Consultancy, Research Funding. Amin:Marathon Pharma, Pfizer, BMS , J&J, Boeheinger-Ingelheim, Otsuka: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal