Abstract
Introduction Febrile neutropenia (FN) is a dose-limiting toxicity of myelosuppressive chemotherapy that has been associated with decreased chemotherapy relative dose intensity (RDI) and increased morbidity and mortality. In clinical trials, primary prophylaxis (PP) with recombinant human granulocyte colony-stimulating factors (G-CSFs) has been shown to reduce the risk of FN, chemotherapy dose delays/reductions, decreased RDI, antibiotic use, and FN-related hospitalization. This systematic review and meta-analysis assessed the relative efficacy of PP with different G-CSFs to reduce the incidence of FN in cancer patients who received myelosuppressive chemotherapy in randomized controlled trials (RCTs).
Methods A systematic literature review identified publications (January 1990 to September 2013) of RCTs assessing PP with filgrastim, pegfilgrastim, lenograstim, or lipegfilgrastim versus placebo, no G-CSF PP, or a different G-CSF in adults who received myelosuppressive chemotherapy for solid tumors or non-Hodgkin's lymphoma. Terms for G-CSFs were searched for in PubMed, Embase, Science Citation Index, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment Database, and the National Health Service Economic Evaluation Database; original publications were searched manually. Eligible studies enrolled patients who initiated G-CSF PP 1–3 days after completing chemotherapy in each cycle; control patients were eligible for secondary prophylaxis with the same G-CSF after the first cycle. Study exclusion criteria included granulocyte-macrophage colony-stimulating factor use, leukemia or multiple myeloma, bone marrow or peripheral blood stem cell transplantation, G-CSF for established FN, different doses of the same G-CSF in each treatment arm, and investigational or unapproved drugs. Economic analyses and studies published in languages other than English were excluded. A meta-analysis using mixed-treatment comparison (MTC) was used to calculate the odds ratio (OR) and 95% credible interval of FN in all chemotherapy cycles and in cycle 1 without adjustment for differences in RDI between study treatment arms. No adjustment for differences in RDI between arms was made because RDI was not consistently reported between studies.
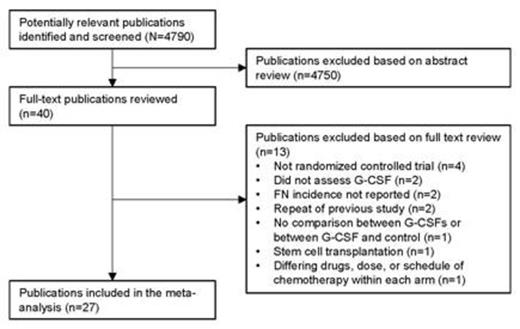
Results Of the 4790 publications initially screened, 27 publications representing 30 studies were included in the meta-analysis (Figure). Over all chemotherapy cycles, there was a statistically significant reduction in the FN risk for PP with filgrastim, pegfilgrastim, lenograstim, and lipegfilgrastim versus no G-CSF PP or placebo, as well as with pegfilgrastim PP versus filgrastim PP (Table). Over all chemotherapy cycles, there was a statistically nonsignificant increase in the FN risk for lipegfilgrastim PP versus pegfilgrastim PP; a statistically significant difference was not expected because of the small sample size (n=306) for lipegfilgrastim (2 studies). During chemotherapy cycle 1, there was a statistically significant reduction in the FN risk for filgrastim PP versus no G-CSF PP or placebo, pegfilgrastim PP versus no G-CSF PP or placebo, and lipegfilgrastim PP versus no G-CSF or placebo (data not shown).
Conclusions Using MTC without adjustment for RDI, PP with short-acting and long-acting G-CSFs was associated with a reduced FN risk in patients receiving myelosuppressive chemotherapy for solid tumors or non-Hodgkin's lymphoma. In future studies, consistent reporting of RDI between study arms is needed to adequately assess the influence of RDI on FN outcomes and to eliminate the potential bias in comparisons between G-CSF arms receiving more intensive chemotherapy than control arms.
| Primary prophylaxis comparisons across all cycles . | Median OR (95% CrI) . |
|---|---|
| Filgrastim vs no G-CSF or placebo (11 studies; n=2181) | 0.42 (0.30–0.57) |
| Pegfilgrastim vs no G-CSF or placebo (5 studies; n=2060) | 0.25 (0.17–0.40) |
| Lenograstim vs no G-CSF or placebo (5 studies; n=467) | 0.34 (0.19–0.60) |
| Lipegfilgrastim vs no G-CSF or placebo (1 study; n=375) | 0.35 (0.14–0.88) |
| Pegfilgrastim vs filgrastim (6 studies; n=647) | 0.61 (0.40–0.98) |
| Lipegfilgrastim vs pegfilgrastim (2 studies; n=306) | 1.39 (0.54–3.50) |
| Heterogeneity, between-trial SD logOR, mean (95% CI) | 0.41 (0.17–0.69) |
| Mean residual difference | 60.01 |
| Primary prophylaxis comparisons across all cycles . | Median OR (95% CrI) . |
|---|---|
| Filgrastim vs no G-CSF or placebo (11 studies; n=2181) | 0.42 (0.30–0.57) |
| Pegfilgrastim vs no G-CSF or placebo (5 studies; n=2060) | 0.25 (0.17–0.40) |
| Lenograstim vs no G-CSF or placebo (5 studies; n=467) | 0.34 (0.19–0.60) |
| Lipegfilgrastim vs no G-CSF or placebo (1 study; n=375) | 0.35 (0.14–0.88) |
| Pegfilgrastim vs filgrastim (6 studies; n=647) | 0.61 (0.40–0.98) |
| Lipegfilgrastim vs pegfilgrastim (2 studies; n=306) | 1.39 (0.54–3.50) |
| Heterogeneity, between-trial SD logOR, mean (95% CI) | 0.41 (0.17–0.69) |
| Mean residual difference | 60.01 |
Wang:Amgen Inc.: Consultancy. Baser:Amgen Inc.: Consultancy. Kutikova:Amgen Inc.: Employment, Equity Ownership. Page:Amgen Inc.: Employment, Equity Ownership. Barron:Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal