Abstract

Background: P53 activation is a major pathway by which normal tissues respond to DNA damaging agents such as chemo and radiotherapy. We have shown that the use of very low dose arsenic (LDA) for 3 days before chemotherapy in animal models temporarily and reversibly suppresses p53 activation for about 5 days. LDA-mediated protection requires functional p53 and thus is selective to normal tissues, as essentially every cancer cell has dysfunctional p53. Arsenic Trioxide (ATO) is currently used to treat acute promyelocytic leukemia at a much higher dose (50-fold higher than the dose we used for suppression of p53). The primary objectives of this trial were to: 1) define the lowest safe dose of ATO that blocks p53 activity in vitro as measured in patients(pts)' peripheral lymphocytes and 2) assess the potential of LDA to decrease hematological toxicity in pts receiving chemotherapy.
Methods: Pts with malignancies other than leukemia who were to receive at least 4 cycles of myelosuppressive chemotherapy given at least 2 weeks apart were eligible. Pts had to have no baseline p53 activation in peripheral lymphocytes but p53 had to be responsive as measured by an in vitro assay. For objective 1, dose escalation was performed at the starting dose of ATO 0.005mg/kg/day for 3 days. For objective 2, ATO 0.005mg/kg /day x 3 was given prior to chemotherapy cycles 2, 4 and 6 only. WBC, HgB, platelet and ANC counts were obtained on chemotherapy days 1 (prior to chemotherapy), 8, 15 and 22. The p53 activation level in peripheral lymphocytes (prior to chemotherapy) was measured on day 1 of each chemotherapy cycle by ELISA essay. For the current analysis for objective 2, we compared only cycles 1 and 2 at days 1, 8, and 15 among the pts who received 3 week cycle chemotherapy regimens. Their p53 activation level on day 1 of cycle 2 was compared to that on day 1 of cycle 1. Pts whose p53 level was lower in cycle 2 were defined as group A (i.e. suppressed). Those whose p53 level was higher in cycle 2 were defined as group B. Groups A and B were compared in log units with regard to four tests (WBC, HgB, platelet, and ANC) with a repeated measures linear model of the test result in terms of group (A,B), test, cycle (1,2), day (1, 8, 15), and interaction terms, assuming an autoregressive order 1 autocorrelation matrix. All statistical testing was two-sided with a significance level of 5%; corrections for multiple comparisons were not applied.
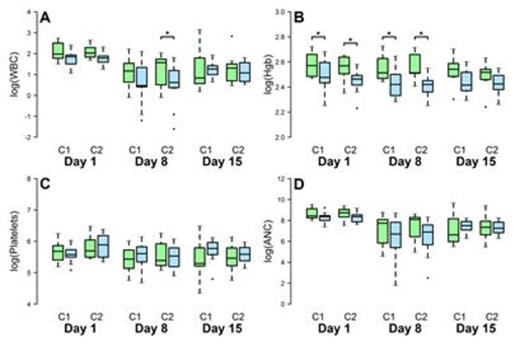
Results: Thirty-three evaluable pts were accrued. Chemotherapy was: TC (docetaxel, cyclophosphamide)-8, AC (doxorubicin, cyclophosphamide)-7, eight other regimens-18 pts. Twenty-four pts were treated with 3 week regimens and have p53 data available. For Objective 1, ATO at a dose of 0.005mg/kg/day for 3 days blocked p53 activity in vitro as measured in pts' peripheral lymphocytes and was not associated with any toxicity. Eight pts belonged to group A and 14 group B. Two pts who showed no change in p53 on day 1 between cycles 1 and 2 were excluded. For Objective 2, we found that the relationship between group and mean test result varied significantly with test (p<0.001) and day (p<0.001), but not with cycle (p=0.38). Group contrasts by day and cycle revealed significantly increased mean log(WBC) in group A compared to group B at day 8 in cycle 2 and significantly increased mean log(HgB) in group A compared to group B at days 1 and 8 in cycles 1 and 2. Similar though not significant increases in mean log(ANC) in Group A relative to Group B were observed in cycle 2 at days 1 and 8. Mean log(platelet counts) did not exhibit any significant or meaningful patterns (See Figure).
Conclusions: Our findings suggest that LDA pretreatment prior to chemotherapy can keep p53 from being activated with chemotherapy in one third of the pts. These pts appear to experience less myelosuppression as manifested by higher WBC and HgB in cycle 2 compared to 1. Increased mean log(HgB) in group A relative to group B at days 1 and 8 even for cycle 1 may imply different biology for pts whose p53 is suppressed vs not suppressed by LDA. Further study is needed to confirm this finding and to develop this new strategy to protect bone marrow during chemotherapy.
Mean test results in log units by group (A: Suppressed-Green, B: Activated- Blue), cycle (1, 2) and day (1, 8, 15) for WBC (panel A), HgB (panel B), Platelets (panel C), and ANC (panel D). Pts received pretreatment with ATO before cycle 2. Significant group differences (p<0.05) are indicated with asterisks.
Mean test results in log units by group (A: Suppressed-Green, B: Activated- Blue), cycle (1, 2) and day (1, 8, 15) for WBC (panel A), HgB (panel B), Platelets (panel C), and ANC (panel D). Pts received pretreatment with ATO before cycle 2. Significant group differences (p<0.05) are indicated with asterisks.
Ha:ASPECT Oncology: Research Funding. Yuan:ASPECT Oncology: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal