Abstract
Background: CD20 antigen, expressed on greater than 90% of B-cell lymphomas, is an established target for antibody therapy with monoclonal antibodies like Rituximab, which can selectively deplete CD20-expressing cells in peripheral blood and lymphoid tissues. Understanding properties and interactions of CD20 antigens is quintessential for developing better targeted agents and overcoming resistance. It has not been clearly established yet whether CD20 antigen density corresponds with response to Rituximab. Flow cytometry is still the most widely used technique to detect CD20 level in human serum which is expensive, time consuming and does not reveal any details of interaction between the molecules. We have developed a new innovative biosensor based novel technique to study real time interaction of CD20 antigens with Rituximab using QCM Piezo-immunosensor. This quantitative label free peptide based assay can be used to characterize cell surface antigen, to study antigen- antibody interactions and obtain understanding of mechanisms of resistance to therapy.
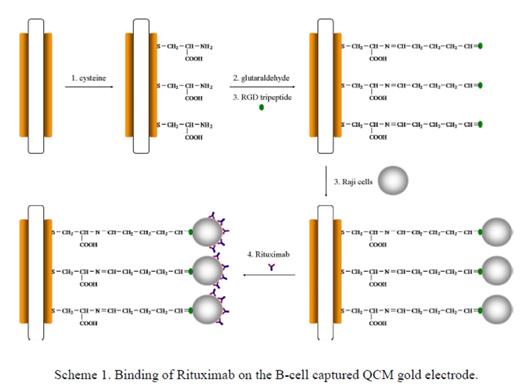
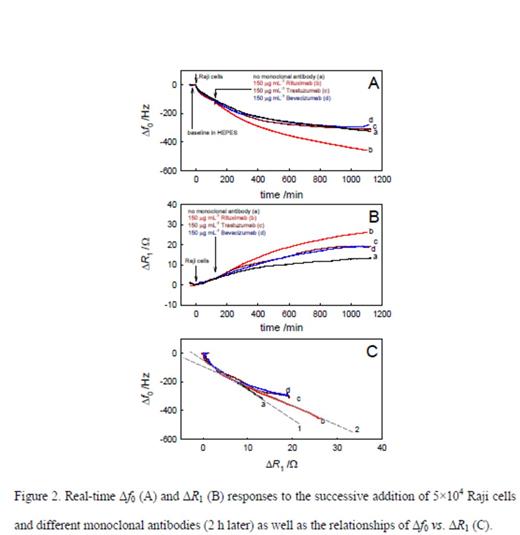
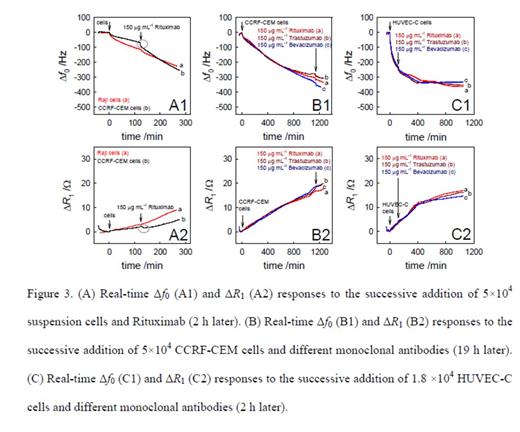
Method: The immobilization of Raji cells on the QCM gold electrode surface using RGD tripeptide was electrochemically confirmed (Figure 1). The real-time processes of attachment of Raji cells on the gold electrode and the subsequent binding of Rituximab to the cells were studied using QCM biosensor. The interaction between Rituximab and Raji cells led to the increased resonant frequency shifts (df0) in the studied antibody concentration range from 5 to 250 μg mL-1 following the Langmuir adsorption model (Figure 2). From these observations, the apparent binding constant between a single-layer of Rituximab and Raji cells was calculated to be 1.6×106 M-1 (Figure 3). Control experiments using other therapeutic antibodies (i.e., Trastuzumab and Bevacizumab) and different cells (i.e., T cells and endothelial cells) proved very specific interaction between Rituximab and CD20 antigens on B cells. Calcium and Manganese ions were added to the cell culture and corresponding responses by QCM were monitored.
Results: CD20 binding with Rituximab was very specific. This binding decreased the electrochemical activity and stability of the cells, supporting the cell lysis mechanisms of action of Rituximab. We have shown a systematic approach for using QCM technique to quantify the apparent binding constant between Raji cells and Rituximab which can reveal CD20 antigen density. Moreover, increased QCM responses were found in the presence of Ca2+ ions and high concentration Mn2+ ions, supporting the function of CD20 as a calcium ion channel. Microscopic inspection proved that increased Ca2+ ions could promote the Rituximab binding and cell lysis induced by Rituximab, which was not seen with Manganese.
Conclusion: CD20 antigen density and interactions of CD20 antigens with respective monoclonal antibodies will help physicians to determine the clinical efficacy and resistance mechanisms to targeted antibodies like Rituximab and Ofatumumab. For the first time, we have established a low cost, highly sensitive, fast, synthetic, QCM assay which could be used as a basis for developing a new generation of affinity-based Immunosensor assays. This real time capability of QCM and its simplicity of operation are highly suitable for multipurpose studies on living cells including cell immobilization, cytotoxicity of drugs, and the cell action mechanisms.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal