Abstract
Introduction: Combination therapy for longer periods but at low dose, also called metronomic scheduling, might be an effective manner to treat patients with relapsing myeloma. In particular if the used agents attack the malignant clone in an alternative manner. Therefore we used the combination of bortezomib, dexametasone and daily low dose of oral cyclophosphamide as an induction regimen followed by one year of maintenance therapy consisting of bortezomib and cyclophosphamide.
Methods: Relapsing myeloma patients, bortezomib naïve, were treated with three cycles of 1.3 mg/m2 bortezomib at day 1, 4, 8 and 11, cyclophosphamide 50 mg daily, and 20 mg dexamethasone at day 1, 2, 4, 5, 8, 9, 11 and 12 followed by three cycles of bortezomib 1.6 mg/m2 (day 1, 8, 15 and 2), cyclophosphamide (50 mg) daily and dexamethasone (20 mg) at day 1, 2, 8, 9, 15, 16, 22 and 23. Maintenance therapy consisting of bortezomib 1.3 mg/m2 every two weeks and daily dose of 50 mg cyclophosphamide for one year was applied to patients in partial or complete remission. Primary endpoints were toxicity during re-induction and maintenance therapy. Secondary endpoints were response to treatment and progression free and overall survival.
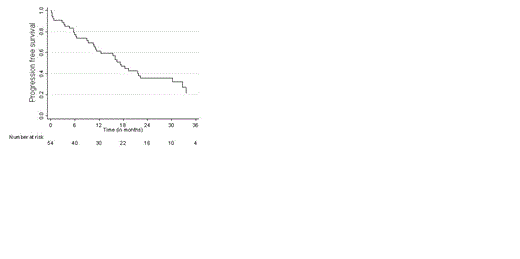
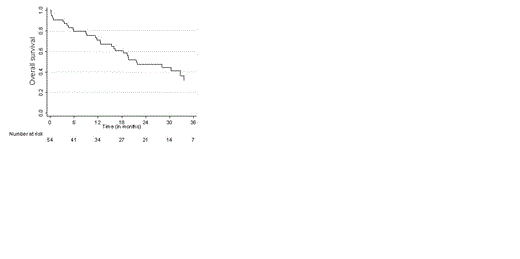
Results: 59 patients with relapsing multiple myeloma were included of whom 69% were in first relapse (Table 1). The upfront treatment consisted mainly of thalidomide-based and vincristine-based chemotherapy and 40% of the patients have been treated with an autologous stem cell transplantation. All 6 cycles of induction chemotherapy could be given in 49% of the patients. Premature discontinuation before starting maintenance therapy was due to toxicity (31%), progressive disease (7%), death (7%) or other reasons (6%). Myelosuppression was the most common side effect with WHO grade 3-4 in 31% of the patients. Neuropathy grade 3-4 was observed in 16% of patients, partially due to the fact that bortezomib was given intravenously during the first 2 yrs of the protocol which included 76% of the patients. Maintenance therapy was started in 47% of the patients with a median duration of 7.3 months (range 0.36.-13.4). Grade 3-4 toxicity was observed in 25% of the patients including infections (n=3) and myelosuppression (n=3) which did not resulted in discontinuation of therapy. Median follow up time was 29 months with an overall response of 62%, and a very good partial response (VGPR), complete remission (CR) in 21% and 7% of the patients respectively. During the maintenance phase an improvement in responsiveness was observed in 25% of the patients. The CR rate increased with 9% to a total of 16%. VGPR rate was 20% and 16% of the patient had a PR. At end of the maintenance therapy 50% of patients started with maintenance had stable disease. The median progression free survival (PFS) was 17.2 months (range 0.13 – 43.5) as depicted in figure 1. and the median overall survival was 21.6 months (range 0.46-54.4, figure 2). During follow up 33 % of the patients died due to progression of MM.
Conclusion: The present study demonstrates that combination therapy with bortezomib, continuous low dose cyclophosphamide and dexamethasone is an effective and manageable regimen. Adding a year of maintenance was feasible with limited side effects and an increase in CR rate.
patient characteristics
| . | Patients (%) . |
|---|---|
| Age, mean (min,max) | 69 (46-86) |
| Sex | |
| Male | 56 |
| Female | 44 |
| Relapse number | |
| First relapse | 75 |
| Second relapse | 20 |
| Third relapse | 5 |
| Performance status | |
| 0 | 65 |
| 1 | 29 |
| 2 | 5 |
| M-protein heavy chain | |
| IgA | 18 |
| IgG | 65 |
| Light chain disease | 18 |
| Polyneuropathy | |
| No | 61 |
| Yes | 39 |
| . | Patients (%) . |
|---|---|
| Age, mean (min,max) | 69 (46-86) |
| Sex | |
| Male | 56 |
| Female | 44 |
| Relapse number | |
| First relapse | 75 |
| Second relapse | 20 |
| Third relapse | 5 |
| Performance status | |
| 0 | 65 |
| 1 | 29 |
| 2 | 5 |
| M-protein heavy chain | |
| IgA | 18 |
| IgG | 65 |
| Light chain disease | 18 |
| Polyneuropathy | |
| No | 61 |
| Yes | 39 |
Progression free survival
Overall survival
Waal de:Jansen Cilag: Research Funding. Munck de:Jansen Cilag: Research Funding. Woolthuis:Jansen Cilag: Research Funding. velden Van Der:Jansen Cilag: Research Funding. Tromp:Jansen Cilag: Research Funding. Hoogendoorn:Jansen Cilag: Research Funding. Vellenga:Jansen Cilag: Research Funding. Hovenga:Jansen Cilag: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal