Abstract
Introduction
Despite progress in chronic lymphocytic leukemia (CLL) treatment, new therapies are needed especially for relapsed/refractory (R/R) patients (pts). BCL2 is an anti-apoptotic protein expressed at high levels in all cases of CLL. GDC-0199 is an oral, highly selective BCL2 inhibitor. Clinical data for GDC-0199 have shown promising anti-CLL activity. Obinutuzumab (Gazyva®, Gazyvaro™) is a Type II, glycoengineered anti-CD20 antibody that has increased direct cell death, and enhanced antibody-dependent cell-mediated cytotoxicity. Obinutuzumab and chlorambucil have demonstrated improved progression-free survival compared to rituximab and chlorambucil in previously untreated pts with CLL. Also, preclinical data suggest that GDC-0199 combined with obinutuzumab may show synergistic activity in CLL. Collectively, these data support combining GDC-0199 and obinutuzumab for pts with CLL. We present data from an ongoing phase 1b study that is evaluating the safety and tolerability of GDC-0199 in combination with obinutuzumab in R/R or previously untreated pts with CLL.
Methods
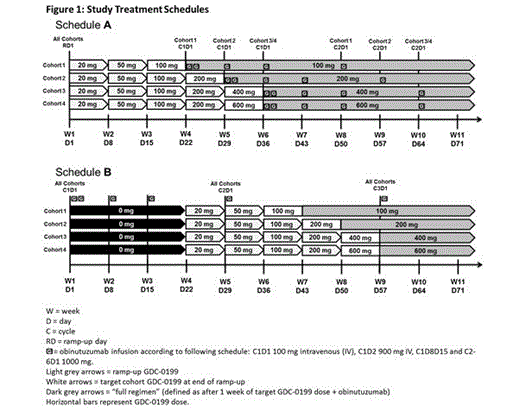
Pts with an ECOG PS ≤1, adequate marrow, hepatic, renal and coagulation function are enrolled in a 3+3 study design with cohorts ranging from 100 to 600 mg/day of GDC-0199. Study eligibility is not restricted by cytogenetics or CLL risk profile. Study drug administration incorporates a gradual dose ramp-up of GDC-0199 to reduce the risk of tumor lysis syndrome (TLS), and staggering of the two agents. Pts are assigned to one of two dosing schedules (Figure 1) with GDC-0199 (Schedule A) or obinutuzumab (Schedule B) introduced first. After completing combination therapy, R/R pts continue single-agent GDC-0199 until disease progression. Adverse events (AEs) are graded according to NCI-CTCAE v.4 criteria. Dose-limiting toxicities (DLTs) are identified during the first 21 days of combination treatment and focus on potential AEs of TLS, infusion related reactions (IRRs), and cytopenias.
Results
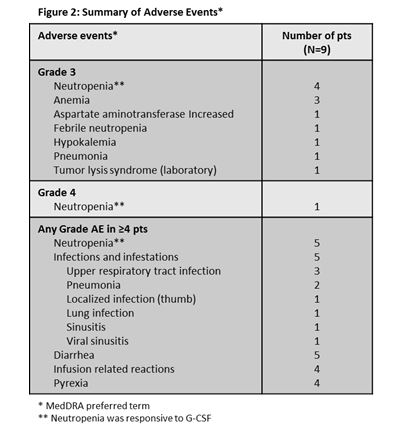
As of May 2014, 9 R/R pts are on the dose finding stage of the study; 4 additional R/R pts were enrolled and discontinued following clinical TLS events in other GDC-0199 studies. No clinical TLS was observed in these 4 pts. The data presented here describe the 9 pts who continue on study treatment. Pts were assigned to 1 of 3 TLS risk groups based on screening ALC and tumor bulk: low risk 0 pts, medium risk 4 pts, and high risk 5 pts. Median time on study was 98 (range 7-252) days. No DLTs were observed in the 3 pts enrolled in the 100 mg GDC-0199 dosing cohort. Six pts were enrolled in the 200 mg GDC-0199 dosing cohort due to expansion following a DLT of laboratory TLS (characterized by asymptomatic laboratory abnormalities in potassium and phosphate) observed in 1 of the first 3 pts. Baseline characteristics include: median age 69 (range 59-80) years, 6 male pts, median of 4 prior CLL therapies (range 1-6), beta-2 microglobulin of ≥3.5 mg/L in 7 of 8 pts with available data, and IGVH mutation in 1 of 7 pts with available data. Cytogenetic data are available for 4 pts: none had del17p, 1 pt had del 11q, 1 pt had trisomy 12 and 2 pts had del 13q. The most common AEs included neutropenia (Figure 2). Dose interruptions of GDC-0199 or obinutuzumab in response to AEs were observed in 5 pts (2 pts had dose interruptions for obinutuzumab only [IRRs], and 3 pts had dose interruptions for GDC-0199 [mainly electrolyte abnormalities and cytopenias] and obinutuzumab [IRRs]); 1 pt in the 100 mg GDC-0199 dosing cohort had a dose reduction to 50 mg per day after 2 cycles of combination therapy due to ongoing neutropenia, and subsequently completed 6 cycles of combination treatment. IRRs were limited to the first infusion of obinutuzumab and were of Grade ≤2. One event of Grade 3 pneumonia required hospitalization. No treatment emergent bleeding events or deaths occurred on study.
<![if !vml]><![endif]>
Conclusion
This is the first study combining GDC-0199 and the novel anti-CD20 antibody obinutuzumab in CLL and suggests that the combination is safely administered at the doses given. Prophylactic measures and a gradual dose ramp-up of GDC-0199 appear to reduce the incidence of TLS. Despite 9 pts being identified as medium or high risk for TLS, only 1 developed laboratory TLS, which was transient and managed. No clinical TLS was observed in these 9 pts. Dose escalation continues in R/R pts at 400 mg/day of GDC-0199. Schedule B and previously untreated pts will be enrolled in the near future.
Flinn:Genentech: Research Funding. Brunvand:Genentech: Speakers Bureau. Hillman:Roche Pharmaceuticals: Honoraria, Research Funding. Jones:Genentech: Advisory Board Other. Lymp:Genentech: Employment. Elhamy:Genentech: Employment. Vosganian:Genentech: Employment. Huang:Genentech: Employment. Kipps:Cegene, Pharacyclics, AbbVie, Genentech: Advisory Board Other, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal