Abstract
Background and Significance: Cell-cell interactions between blood cells and endothelial cells play an important role in sickle cell disease (SCD) pathophysiology. While in vivo transgenic animal models and in vitro systems have both contributed to our understanding of these pathologic cell-cell interactions in SCD, isolating the causes and effects of cellular interactions is exceedingly difficult in the former and recapitulating the complex vascular geometries found in vivo is not readily available with current systems in the latter. The vascular system comprises diverse geometries that range from normal (e.g. curves and bifurcations) to pathologic (e.g. aneurysms and stenoses) and as blood flows from one vascular geometry to another, the local shear stress profile acutely changes. Interestingly, changes in shear stress are known to alter endothelial pro-inflammatory signaling pathways and expression of cell adhesion molecules, especially vascular cell adhesion molecule-1(VCAM-1) (Tzima, Nature, 2005), which is implicated in SCD vasculopathy. Here we present a rapid and inexpensive method using only off-the-shelf materials to create “do-it-yourself” (DIY) microfluidic devices that incorporate endothelial cells and clinically relevant vascular geometries; this system effectively and bridges current in vitro and in vivo models to study SCD. Using this technique, we developed a vascularized bifurcation, and observed that shear stress changes can be extremely localized, affecting only several 10s of cells, and are associated with changes in VCAM1 expression. We used this in vitro vascularized bifurcation to test the hypothesis that SS RBC-endothelial cell adhesion occurs primarily at bifurcations, which are difficult to visualize in vivo (Nagel, Arterioscler Thromb Vasc Biol, 1999). We demonstrate that SCD RBCs do primarily aggregate at bifurcations, specifically in locations where the shear stress has decreased and VCAM-1 is upregulated.
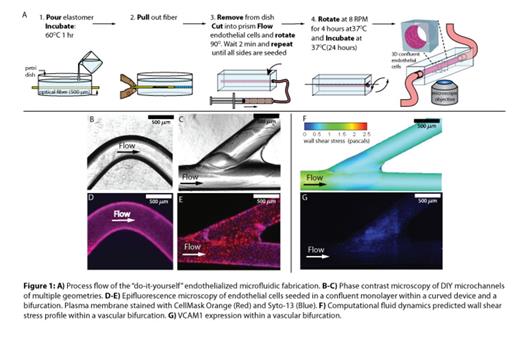
Methods: In order to bridge in vitro data with the complex vascular geometric environments found in vivo, we developed a “DIY” endothelialized microfluidic model (Figure 1A). A strand of 500um diameter polymethylmethacrylate (PMMA) optical fiber is laid flat on top of a layer of polydimethylsiloxane (PDMS) and covered with a second, thin layer of PDMS. After curing, the optical fiber is pulled out, exposing a hollow, circular, channel that can be used as a microchannel to seed endothelial cells. A wide variety of endothelial cells can be successfully seeded in these devices, such as human umbilical vein endothelial cells, human aortic endothelial cells, and human microvascular endothelial cells. Slight alterations to this fabrication method result in the creation of multiple vascular geometries, such as curved or bifurcated channels with or without aneurysms or stenoses.
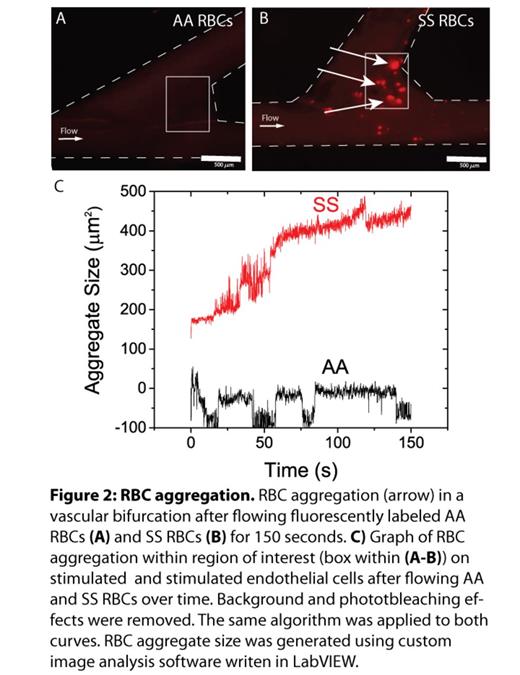
Results: Curved channels & bifurcations (Figure 1B-C) are seeded with endothelial cells (Figure 1E-F). Computational fluidic dynamics calculations show that the shear varies by 2.5 fold within the bifurcation. As shear affects endothelial expression, we tested if the extremely localized shear changes created in this system were sufficient to alter local endothelial expression of VCAM-1 Indeed, in our system, VCAM1 expression significantly correlated with shear variation (Figure1G), and was highest near the bifurcation point. Noting this localized variation in adhesion molecule expression, we tested whether the bifurcations are implicated in SCD RBC adhesion to the endothelium. With our vascularized bifurcation model and custom image analysis software that quantifies RBC aggregation, we observed that SCD RBC adhesion predominantly occurred at the point of bifurcation where the shear is lowest and VCAM1 expression is greatest, and minimal endothelial adhesion occurred with healthy control RBCs (Figure 2). This phenomenon persisted with tumor necrosis factor-stimulation of the endothelium.
Conclusion: This DIY system represents an easily accessible technique that allows any researcher to bridge the gap between in vitro and in vivo models of pathological cell-cell interactions in SCD. We demonstrate that recapitulating the complex vascular geometries in vivo is vital to understanding blood cell-endothelial interactions and this system will not only be useful for studying SCD, but a myriad of hematologic and vascular diseases as well.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal