Abstract
Introduction: Tyrosine kinase inhibitor (TKI) resistance remains a major impediment to successful treatment of patients with chronic myeloid leukemia (CML). The 3rd generation TKI ponatinib (Pon), has been demonstrated to successfully overcome BCR-ABL1 kinase domain (KD) mutation based resistance including BCR-ABL1T315I, which inhibits the binding of all other available TKIs. Pon has demonstrated efficacy in CML patients who acquired resistance to other TKIs. However, little is known about the mechanisms which result in resistance to Pon. Here, modes of Pon resistance have been investigated invitro in both TKI naïve and treated BCR-ABL1+ cell lines.
Methodology and Results: Pon resistance was generated in 4 BCR-ABL1+ cell lines 1) K562, 2) K562 DOX (increased ABCB1) 3) K562 DOX 55D –resistant to 55 nM dasatinib (Das) and 4) K562 T315I – T315I mutation developed in the presence of Das. Both Das treated cell lines have 3-4 fold higher expression of BCR-ABL1 mRNA compared to control lines. The K562 T315I line contains 44% T315I mutation whereas no KD mutation was found in the other lines. Parental Pon naive controls were maintained in parallel.
Resistant cell lines (Table 1) were established by exposing the above cell lines to increasing concentrations of Pon for 660-900 days. The K562 T315IR resistant line demonstrated a >17 fold increase in IC50Pon compared to naïve control. While no expression of ABCB1 or ABCG2 was observed, BCR-ABL1 transcript level was raised 7 fold with a concomitant 4 fold increase in pBcr-Abl by western blot. In addition, the level of T315I in the resistant line increased from 44% to 81%, suggesting that increasing T315I% together with Bcr-Abl upregulation were the major resistant mechanisms in this line.
The K562 DOX 55DR line demonstrated transient increases in BCR-ABL1 that reduced to basal levels concomitant with the development of the compound mutations G250E (71%) and E255K (64%). The IC50Pon was increased 47 fold compared to control, and high Das and imatinib (Im) IC50s confirmed the resistance to other TKIs (IC50Das: 1350 nM and IC50Im: 260 μM).
In contrast, the Das naïve K562R and K562 DOXR resistant lines did not have demonstrable KD mutations. While 7AAD and annexin V staining confirmed Pon resistance, the IC50Pon was not higher in K562R and only 2 fold higher in K562 DOXR compared to controls, suggesting resistance to Pon was likely mediated by a Bcr-Abl independent mechanism. This was supported by a 2 fold reduction in pBcr-Abl and pSTAT5 in the Pon resistant lines compared to controls. Therefore, the expression levels of alternative pathway molecules were examined to determine possible mechanisms.
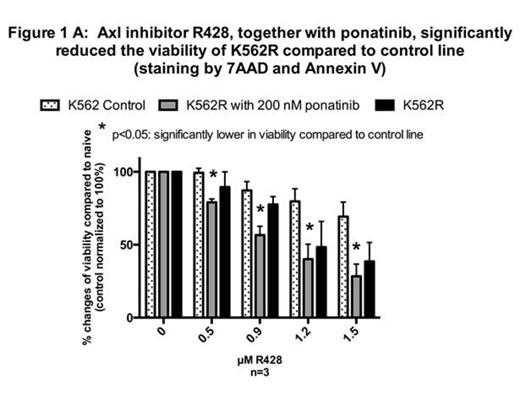
Axl, a receptor tyrosine kinase has been previously associated with TKI resistance. A 7 fold increase in AXL mRNA was found in K562R line, and the K562 DOXR line demonstrated increased Axl protein. Importantly, after 72 hours incubation with Pon and the Axl inhibitor, R428, the viability of the two resistant lines were significantly reduced (Fig 1), suggesting Axl is likely critical in mediating Pon resistance. Furthermore, increased Axl was associated with increased cell adhesion in both lines, and adherence is a hallmark of Axl expression.
Conclusion: As Pon binds to native Bcr-Abl stronger than to Bcr-AblT315I, an increased proportion of T315I reduced sensitivity to Pon in vitro. Pon exposure in a cell line predisposed to KD mutations resulted in the development of the compound mutations G250E and E255K, which rendered the cells insensitive to all tested TKIs. Interestingly the mode of resistance in the two Das naïve lines appears Bcr-Abl independent, and is likely mediated by Axl. While limited to in vitro studies these findings suggest that in the setting of prior TKI exposure, mutations are likely to be a primary form of Pon resistance. In the TKI naïve setting Bcr-Abl independent modes of resistance may be more likely suggesting combination therapeutic approaches may be warranted to overcome or prevent Pon resistance in the high risk setting.
Summary of resistance mechanisms detected in 4 cell lines.
| . | Pon culture condition (nM) . | Pon resistance Bcr-Abl dependent/ independent . | BCR-ABL1 mRNA (fold ↑ ) . | pBcr-Abl & pSTAT5 (fold ↑ ) . | KD mutation . | IC50Pon (fold ↑ ) . | AXL mRNA (fold ↑ ) . | ↑ cell surface Axl . | Adherence . |
|---|---|---|---|---|---|---|---|---|---|
| K562 T315IR | 100 | DP | 7 | 4 | T315I | >17 | 5 | ✖ | ✖ |
| K562 DOX 55DR | 200 | DP | 1 | 1 | G250E E255K | 47 | ✖ | ✖ | ✖ |
| K562R | 200 | IDP | 1 | 0.5 | ✖ | ✖ | 7 | ✖ | ✓ |
| K562 DOXR | 200 | IDP | 3 | 0.5 | ✖ | 2 | ✖ | ✓ | ✓ |
| . | Pon culture condition (nM) . | Pon resistance Bcr-Abl dependent/ independent . | BCR-ABL1 mRNA (fold ↑ ) . | pBcr-Abl & pSTAT5 (fold ↑ ) . | KD mutation . | IC50Pon (fold ↑ ) . | AXL mRNA (fold ↑ ) . | ↑ cell surface Axl . | Adherence . |
|---|---|---|---|---|---|---|---|---|---|
| K562 T315IR | 100 | DP | 7 | 4 | T315I | >17 | 5 | ✖ | ✖ |
| K562 DOX 55DR | 200 | DP | 1 | 1 | G250E E255K | 47 | ✖ | ✖ | ✖ |
| K562R | 200 | IDP | 1 | 0.5 | ✖ | ✖ | 7 | ✖ | ✓ |
| K562 DOXR | 200 | IDP | 3 | 0.5 | ✖ | 2 | ✖ | ✓ | ✓ |
✓= yes; x = no; ↑=increased; DP = dependent; IDP = independent.
Hughes:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. White:Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Ariad: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal