Abstract
Lymphoma immunotherapy can result in durable immune and clinical responses. Nevertheless, the clinical impact of such therapy remains suboptimal and there is still a need to apply our growing understanding of cancer immunity to the design and testing of rational combination approaches to lymphoma immunotherapy. This includes consideration of the three steps necessary to generate an antitumor response: (1) providing tumor antigens to dendritic cells (DCs) in a manner that enhances their ability to process and present antigens to T cells; (2) enhancing T cell activation; and (3) overcoming immunosuppression so that activated anti-lymphoma T cells can proceed unrestrained.
In situ immunization generates systemic immune responses through local injection of agents into the tumor, thus providing DCs with the patient’s own tumor antigens. We evaluated a three-step approach to in situ immunization against lymphoma using Doxorubicin, anti-CTLA-4 and anti-OX40: (1) Doxorubicin (Dox) to induce the expression of “eat-me” signals by dying tumor cells, facilitating their phagocytosis by DCs; (2) anti-OX40 antibody to augment OX40-mediated stimulation of T cells; (3) anti-CTLA-4 antibody to block immunosuppression imposed by CTLA-4 on T cells. While Dox is highly effective as a systemic anti-lymphoma agent and has been reported to induce immunogenic cell death, intratumoral injection of soluble Dox is not clinically feasible due to its strong vesicant effect. Poly(lactide-co-glycolide) (PLGA) is an FDA-approved polymer that is used in biodegradable surgical sutures and microparticles (MPs) with sustained-release properties. Thus, PLGA MPs loaded with Dox (Dox MPs) represent a clinically translatable approach for delivering Dox, as its slow release would decrease likelihood of vesication. In addition, PLGA MPs at an optimal size of 1-µm enhance antigen-specific immune responses by activating the NALP3 inflammasome in DCs.
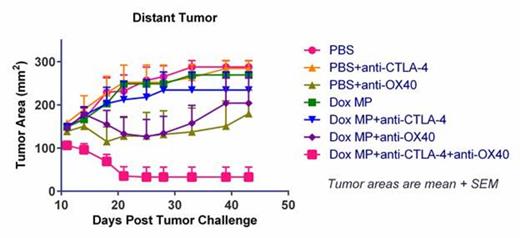
While both tumor cells and DCs are exposed to Dox released by MPs in the tumor, we found in vitro that Dox MPs (1-µm) are less cytotoxic to DCs than to A20 B-lymphoma cells and do not require internalization for their cytotoxic activity. Dox MPs significantly enhanced phagocytosis of A20 by DCs as compared to soluble Dox. In vivo, we used the two-tumor mouse model to assess immune responses. This allowed us to monitor the local effect (on the tumor injected with MP) and the systemic effect (on the distant tumor that was not injected with MP) of therapy. Dox MPs injected intratumorally do not induce vesication even at doses as high as 100 µg Dox. Using a low dose of Dox MPs (2 µg Dox) and of antibody to limit systemic toxicity, we found that three-step therapy induced CD4- and CD8- T cell-dependent systemic immune responses that enhanced T cell infiltration into distant tumors. This led to their eradication and significantly improved survival as compared to antibody-only therapy (87% of mice treated with all three components became tumor-free). Moreover, all three components were required for maximum efficacy (Figure 1). These results demonstrate that systemic antitumor immune responses can be generated locally by three-step therapy. The potential value of this approach to immunotherapy is not limited to lymphoma and merits further evaluation in lymphoma and other cancers.
Mice challenged with two A20 tumors were treated with PBS (control) or Dox MP into one tumor and antibodies (anti-CTLA-4 and/or anti-OX40) given systemically.
Mice challenged with two A20 tumors were treated with PBS (control) or Dox MP into one tumor and antibodies (anti-CTLA-4 and/or anti-OX40) given systemically.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal