Abstract
Background: Despite an increase in our understanding of the pathophysiology of indolent non-Hodgkin lymphoma(iNHL) and the recent advances in targeted therapies, the optimal treatment for patients has not been determined. Rituximab has demonstrated clinical benefit in combination with chemotherapy for indolent NHL, and rituximab-based chemotherapy combinations are used both in first-line and salvage therapy. The immunomodulatory agent, lenalidomide, has demonstrated clinical activity as a single agent and in combination with rituximab in most NHL subtypes.
Aims: The goals of this study were to determine the clinical benefit, safety profile, and immunological effects associated with response to R2 in patients with previously untreated iNHL.
Methods: Patients with previously untreated iNHL with measurable disease and an ECOG Performance Status Score ≤ 2 were enrolled. Oral lenalidomide (20 mg/day) was given on days 1–21 of a 28-day cycle, with 4 weekly doses of rituximab (375mg/m2) as an induction phase. This was followed by lenalidomide maintenance that was continued until disease progression or unacceptable toxicity. Overall response rate (ORR) was the primary endpoint, and secondary endpoints included response duration, overall survival, progression-free survival (PFS), immune correlates and safety. For immunology studies, plasma and viable PBMC were obtained at baseline, day 15, day 30, day 60 and day 120. Levels of plasma IL-1β, 2, 6, 8, 10, 12, GM-CSF, IFN-γ, TNF-α and CXCL-10 (IP-10) were measured by a multiplex assay (Meso Scale Discovery). T cell activation (CD38+/HLA-DR+) and expression of PD-1 markers on CD4+ and CD8+ lymphocytes were examined by flow cytometry assays using viable cryopreserved PBMCs. Th1, Th2 and Th17 cells were examined after in vitro stimulation. NK cell activity was examined after in vitro culture with PMA. Subsets of dendritic cell (DC) subsets (CD11c+ and CD123+) were also examined.
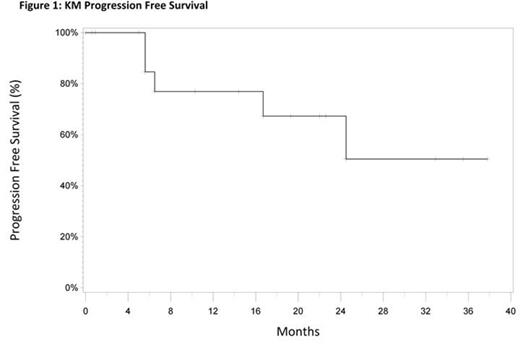
Results: Twenty-two patients are enrolled and 18 are currently evaluable for response assessment. The median age is 60 years (50–78). The histological subtypes included: 19 patients with follicular lymphoma (FL) and 3 with marginal zone lymphoma. Responses were achieved in 17 patients (94%), and 10 (62%) had a CR. At a median follow-up time of 27 months, the median PFS for all patients has not yet been reached (Figure 1). Ten patients (62%) needed at least one dose reduction and 2 patients came off-study due to toxicity (rash). Fatigue (13%), neutropenia (19%), lymphopenia (25%), and hyponatremia (19%) were the most common grade 3 or 4 adverse events. Immunologic examinations indicated a marked increase in D15 IFN-γ and IP-10 levels in patients achieving CR. High baseline levels of TNF-α, IL-10, and IL-1β predicted a poor outcome. Further analyses of immune correlates are currently ongoing, including T cell activation, NK cell functions, DC subsets, and Th1/Th2/Th17/Treg subsets. The results of immune correlates in this trial were compared with those obtained in a trial of equivalently treated patients with relapsed/refractory (R/R) iNHL.
Conclusion: These results demonstrate that the R2 combination has significant clinical activity in patients with previously untreated NHL, with an ORR of 94%, a CR rate of 62% and a median PFS that has not been reached after 27 months. Toxicities were mild and manageable. Immunological studies in this trial, as well as in the trial with R/R patients indicated that lenalidomide-mediated induction of IP-10 and IFN-γ predicted CR and high baseline levels of TNF-α, IL-10, and IL-1β were associated with a poor outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal