Abstract
Background
MCL is characterized by a dismal long term prognosis with a median overall survival of 3-5 years. The addition of rituximab to induction chemotherapy improved significantly response rates of patients with MCL. MR treatment for these patients has the potential to further improve disease control and overall survival (OS). The few trials that addressed that question showed inconsistent results.
Aims
We performed a systematic review and meta-analysis of RCTs in order to assess the effect of MR on clinical outcomes of patients with MCL.
Methods
We included RCTs that compared MR to no treatment or other treatment for patients with MCL, either in first line or relapsed disease. In March 2014 we searched The Cochrane Library, MEDLINE, conference proceedings, and databases of ongoing trials. Two reviewers independently assessed the quality of the trials and extracted data. The primary outcome was all cause mortality. Secondary outcomes included progression free survival (PFS) and infectious adverse events. Relative risk (RR) for dichotomous data and hazard ratio (HR) for time to event datawere estimated and pooled using random-effects model.
Results
We identified 3 trials, conducted between the years 1998 to 2010 and randomizing 434 adult patients with MCL. Most patients were males, with a median age ranging from 61 to 70 years, and predominantly good performance status. Seventy-nine percent of the patients received their first line of treatment. MIPI score was reported in one trial. Induction therapy included rituximab in all three trials. In two trials additional chemotherapy induction was applied consisting of fludarabine, cyclophosphamide (FC) and mitoxantrone in one trial (Forstpointner, Blood 2006), and cyclophosphamide, vincristine, adriamycin, prednisone (CHOP) or FC in the other (Kluin-Nelemans, NEJM 2012); in the third trial rituximab was given alone (Ghielmini, JCO 2005). The control group received no maintenance in two trials and interferon alfa in one trial. All included trials are judged at low risk of selection bias, none were blinded.
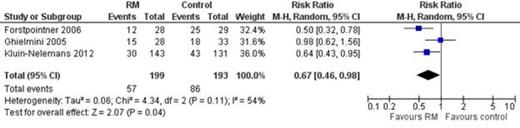
Mortality rate decreased with MR compared to no MR or interferon alfa RR 0.67, 95% CI 0.46 to 0.98, I2 of heterogeneity 54%, 392 patients (Figure).
PFS improved with MR compared to no MR or interferon alfa: HR 0.60, 95% CI 0.44 to 0.82, I2 of heterogeneity = 0.
There was no statistically significant difference in infection rate with or without MR (RR 0.80, 95% CI 0.37 to 1.69, 419 patients).
Conclusions
The results of this meta-analysis support a survival benefit of MR in patients with first line or relapsed/refractory MCL who responded to induction therapy. Additionally, there is a significant improvement of PFS benefit of MR. The absence of significant increase of infection rate as opposed to MR in follicular lymphoma may be attributed to the small sample size. Based on these results patients treated for both first line and relapsed/refractory MCL should receive MR after achieving response to induction.
Pooled RR of mortality of patients with MCL who responded to induction and treated with MR compared to observation or interferon alfa.
Pooled RR of mortality of patients with MCL who responded to induction and treated with MR compared to observation or interferon alfa.
Vidal:Roche: unrestricted grant Other. Ghielmini:Roche: Research Funding, Speakers Bureau. Unterhalt:Roche: Travel Support Other. Shpilberg:Roche: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal