Abstract
Background: Vaso-occlusive painful episodes (VOC) is the most common cause of hospitalization in patients with sickle cell disease (SCD) that often requires opioid analgesics and intravenous fluids. However, due to respiratory depression, the use of opioids during these painful episodes has been associated with the risk of development of acute chest syndrome (ACS), the major cause of early mortality in these patients. Nalbuphine is a unique opioid analgesic that effectively treats pain but lacks significant respiratory depression. Previous small scale studies (Pediatr Blood Cancer 2005 and J Pediatr Hematol Oncol 2011) suggested a lower risk of ACS in patients receiving Nalbuphine during VOC when compared with morphine. We sought to compare outcomes and the risk of ACS in pediatric sickle cell patients hospitalized for VOC in relationship to different opioid treatments.
Methods: We used the Pediatric Health Information System (PHIS), an administrative database of children's hospitals in the US. Patients ≤ 21 years of age with SCD from 43 hospitals from 2005-2013 were included in the study. SCD patients who had a primary diagnosis VOC (ICD-9 codes 282.62, 282.64, 282.42 and 282.69) were included. VOC hospitalizations were divided into four different narcotic analgesic groups. In bivariate analyses, we compared patient demographics, treatment details, risk of ACS, length of stay, hospital costs, and readmissions across groups using chi-square tests or Kruskal-Wallis tests as appropriate. Multivariable models accounted for hospital clustering with generalized estimating equations for binary outcomes and generalized linear mixed effects models with random hospital intercepts and an exponential distribution (due to skewness of the data) for continuous outcomes. A p-value <.05 was considered statistically significant.
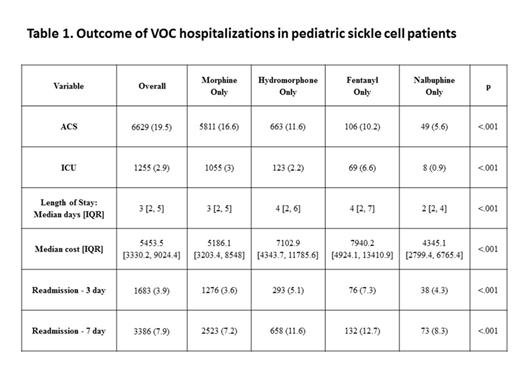
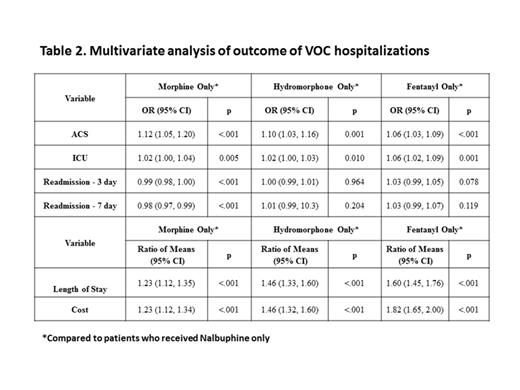
Results: From 2005 to 2013, a total of 11260 unique pediatric SCD patients were identified. These patients had 42688 VOC hospitalizations, and received a single parenteral narcotic analgesic during each hospitalization (Morphine 82.2%, Hydromorphone 13.3%, Fentanyl 2.4% and Nalbuphine 2.1% of VOC hospitalizations). Table 1 describes the outcomes of VOC hospitalizations for all four narcotic groups. In unadjusted analysis, patients who received Nalbuphine only had significantly lower risk of ACS diagnosis (5.6%), ICU admissions (0.9%), shorter median length of stay (2 days) and lower median hospitalization costs ($4345) [Table 1]. Patients who received Morphine had less readmission within 3 and 7 days of discharge after VOC hospitalizations (Table 1). Multivariate analysis after adjusting for age, gender, hospital, season, race, payer source, and complex chronic conditions confirmed that patients who received Nalbuphine had significantly lower risk of ACS diagnosis, ICU admissions, shorter median length of stay, and lower median hospitalization costs compared to patients who received Morphine, Hydromorphone or Fentanyl (Table 2).
Conclusions: In our largest pediatric in-patient sickle cell cohort, Nalbuphine was associated with significantly lower risk of development of ACS when compared with other opioids. In addition, patients received Nalbuphine had significantly lower ICU admissions, shorter hospital stay and lower hospitalization costs suggesting better pain control. However, our study shows that Nalbuphine in this patient population is rarely utilized. Prospective studies are needed to confirm this association and to elucidate the mechanisms that underlie these beneficial effects of Nalbuphine in this patient population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal