Abstract
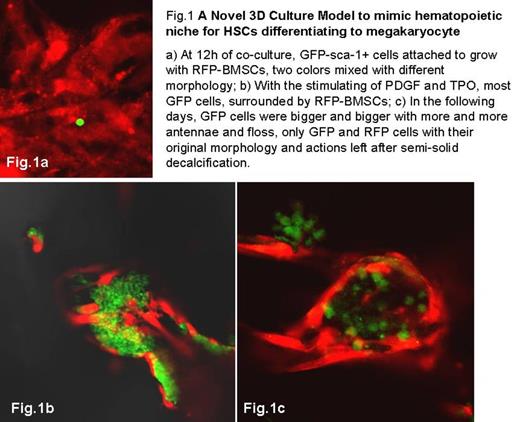
Background: Hematopoietic stem cells (HSCs) are maintained in a particular microenvironment termed as "niche", which are constructed by a number of critical molecules, supporting matrix such as collagen and supporting cells like osteoblasts and bone mesenchymal stem cells (BMSCs), together with physical construct of trabecular bone and endosteum. However, the mechanism of niche is poorly understood since it is surrounded by hard and opaque cortex of bone. Here, we developed a three-dimensional (3D) culture model using the deproteined and degreased human bone or ¦Â-TCP biomaterial, to mimic hematopoietic niche which is highly similar to nature situation and is easy to be observed. Objective: To establish the 3D culture model to mimic the hematopoietic niche with the deproteined and degreased human bone or ¦Â-TCP, where both have an architecture of trabecular bone; the HSC/progeny and supporting cells were gene-marked with GFP and RFP respectively; the process by which the HSCs differentiated to megakaryocyte/platelets were observed within this 3D culture model. Method: 1) 3D culture system: The human bone was obtained by bone biopsy from volunteer donor after the signing of Informed Consent£¬then was deproteined with 30%H2O2 for 2h and degreased with a mixture buffer of Chloroform, methanol and deionized water£¨Vc:Vm:Vw=9:9:2£© in 37¡æ for 24h. ¦Â-tricalcium phosphate (¦Â-TCP) biomaterial was bought from Bio-lu company (France), which is a kind of artificial, mass producible biomimetic macroporous material and clinically proven tissue-engineered bone. Both materials were cut into small pieces with 2-3mm in height and parallel tested. 2) Cells: The C57BL/6 RFP-BMSCs and its complete culture medium were bought from Cyagen Biosciences Inc. (China). Sca-1+ cells were harvested from the bone marrow of GFP transgenic mice by FACS sorting (GFP-sca-1+ cells). RFP-BMSCs and GFP-sca-1+ cells were inoculated into the 3D culture system. 100ng/mL thrombopoietin and 100 ng/mL platelet-derived growth factor (PDGF) were added into co-culture medium for 5 days in 3cm confocal microscopy dishes, both cells were also directly (2D) cultured as control. 3) Observation and Semi-solid decalcification (SSD, 2010 ASH poster, no.2625): The 3D culture system and controls were observed directly by a confocal laser scanning microscope (Olympus), 430nm for GFP and 559nm for RFP. The 3D culture system were also decalcified with self-made SSD and observed to clarify the interaction of GFP and RFP cells. Result: When GFP-sca-1+ cells were co-cultured with RFP-BMSCs for 12h, GFP+ cells attached to grow with RFP-BMSCs, two colors mixed with different morphology (Fig.1a). In the following days, with the stimulating of PDGF and TPO, most GFP cells, surrounded by RFP-BMSCs, were bigger and bigger with more and more antennae and floss (Fig.1b). Hematogenesis happens nearby the architecture of trabecular bone. With the help of SSD, the hard and opaque construction disappeared gradually, only GFP and RFP cells stayed where they were with their original morphology and actions. By frozen section, we can easily distinguish the cell type, developmental stage and interactions between each cell. Discussion: The standard 2D cell culture systems neither reflect the normal architecture, nor material properties such as stiffness, nor the diffusion of soluble factors of the natural system. The deproteined and degreased human bone we used, not only reproduced the spongy architecture of the niche, but also provide a scaffold for cell growth. Furthermore, the self-made SSD can ¡°digest¡± the scaffold but preserve the cells; it is convenient to observe the cells within the ¡°black box¡± in 3D. However, the human bone was hard to obtain. Therefore, we investigated it with ¦Â-TCP, an artificial bone analogs, in parallel. Both materials works well for this novel 3D cell culture model. Conclusion: This novel 3D culture model is a straight-forward and easy-to-observe model in order to understand the bone marrow microenvironment. It is also suitable to study the cell proliferation, differentiation, mobilization and homing, etc.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal