Abstract
Introduction: Leucopenia following myelosuppressive chemotherapy is associated with substantial mortality and costs. Whereas there is a lack of therapeutic options for lymphopenia, in chemotherapy-induced neutropenia, Granulocyte Colony-Stimulating Factor (G-CSF) has proven to be effective in secondary prophylaxis. Despite currently available therapies, mortality due to chemotherapy-induced neutropenia remains high, and safety, tolerability and costs limit the use of available treatment options.
Imidazolyl Ethanamide Pentandioic Acid (IEPA, Myelo001) is a novel small molecule for the treatment of chemotherapy-induced leucopenia. Preclinical and clinical studies have shown that in its oral form IEPA is effective in reducing neutropenia and has antiviral properties without noticeable toxicity.
Objective: To evaluate the effect of IEPA after multiple oral administration in different posologies and at a low (1.0mg/kg) and high dose (100mg/kg) on peripheral components of hematopoiesis in a mouse model of acute cytostatic myelosuppression in comparison to vehicle and Filgrastim (Neupogen¨).
Methods: Twenty-five female CD-1 mice, 6 weeks of age, were randomly assigned to 5 treatment groups: Cyclophosphamide (CPH) (200mg/kg), CPH+Filgrastim (subcutaneous) (0.3mg/kg) (quaque die (q.d.) day (d) 1 to 5), CPH+IEPA (100mg/kg) (q.d., d -1 to 5), CPH+IEPA (100mg/kg) (q.d., d -5 to 5), CPH+IEPA (1.0mg/kg) (q.d., d -5 to 5). The experimental phase started with the determination of baseline white blood cell, red blood cell and platelet counts one day before (pre-) treatment start with IEPA (d -6) and was continued on days 1, 3, 5, 8, 23, 32. A single intraperitoneal (i.p.) injection of Cyclophosphamide (200mg/kg) was administered in all 5 groups on day 0 to induce leuco- and thrombocytopenia. The longitudinal data were analyzed by response profile analysis.1
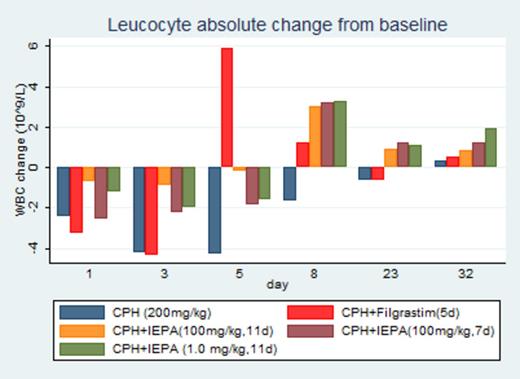
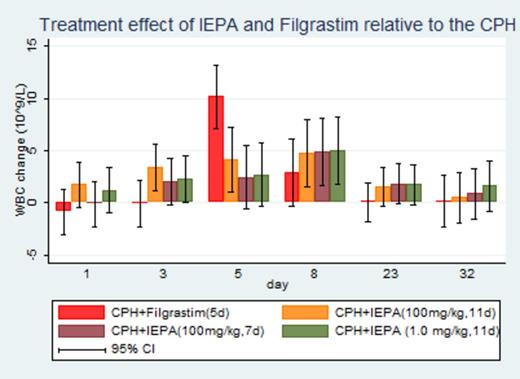
Results: The three groups of IEPA had lower baseline leucocyte counts compared to the CPH+Filgrastim and CPH groups. CPH application caused leucopenia across all 5 groups with the nadir on day 3 to 5. Comparing change from baseline, IEPA significantly reduced leucopenia on days 3, 5, 8 relative to the control group (CPH). No clear dose- or posology-effect relationship was discernible between doses of 1.0 (for 11d), 100 (7d) or 100mg/kg (11d). Compared to Filgrastim, IEPA led to an earlier recovery of leucocytes, followed by a delayed and less pronounced increase in leucocytes above baseline, with the peak of Filgrastim (change of baseline relative to CPH) on day 5 (10.2∙109/L, 95%CI: 7.1 to 13.2∙109/L, p < 0.001) versus day 8 for IEPA (change of baseline relative to CPH) (4.7∙109/L, 95%CI: 1.5 to 8.0∙109/L, p = 0.004 at a dose of 100mg/kg (11d))
Lymphocyte counts change from baseline in all three IEPA groups was numerically higher than in the Filgrastim and CPH arm on day 3, 5 and 8, and day 3 and 8, respectively (not statistically significant). Filgrastim led to a substantial granulocytosis on day 5 (change of baseline relative to CPH) (7.2∙109/L, 95%CI: 5.6 to 8.9∙109/L p < 0.001), whereas a minor increase was observed in the three IEPA groups on day 8. Thrombocytes numbers were slightly increased throughout days 3 to 23 in the IEPA groups, contrasting with a pronounced thrombocytopenia under Filgrastim on day 5. Red blood cell counts decreased after CPH injection (nadir on day 3 to 8), but did not differ among groups.
Conclusion: IEPA demonstrated in 2 doses and posologies a consistent reduction of chemotherapy-induced leucopenia and thrombocytopenia in CD1 mice. Its effect on leucopoiesis and thrombocytopoiesis differs from Filgrastim in a less pronounced early nadir and subsequent lower amplitude of leuco- and granulocytosis. IEPA may offer a new therapeutic option for myelosuppression due to chemotherapy, but requires further preclinical and clinical investigation.
1Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis, Wiley. Hoboken, NJ: Interscience; 2004 p103-140.
Pleimes:Myelo Therapeutics: Employment, Equity Ownership, Managing Director Other; Bayer Pharma AG/ Bayer Healthcare Pharmaceuticals: Consultancy. Flechsig:EPO Berlin-Buch GmbH: Contract Research on behalf and in account of Myelo Therapeutics GmbH Other, Employment. Meyer:Myelo Therapeutics GmbH: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal