Abstract

Aims: Worldwide, rivaroxaban is increasingly used for stroke prevention in atrial fibrillation (SPAF) but little is known about the rates of or reasons for rivaroxaban discontinuation in daily care. Using data from a prospective, non-interventional oral anticoagulation (NOAC) registry, we analysed rivaroxaban treatment persistence.
Methods and results: Persistence with rivaroxaban in SPAF was assessed in an ongoing, prospective, non-interventional registry of >2600 NOAC patients from daily care using Kaplan-Meier time-to-first-event analysis. Reasons for and management of rivaroxaban discontinuation were assessed. Potential baseline risk factors for treatment discontinuation were evaluated using Cox regression analysis.
Between October 2011 and April 2014, 2603 patients were enrolled in the registry. Of these, 1204 (46.3%) received rivaroxaban for SPAF, with 473 (39.3%) switched from VKA pretreatment to rivaroxaban and 731 (60.7%) newly anticoagulated rivaroxaban patients.
As of 30 April 2014, follow-up information was available for all 1204 patients (100%). By that date, the median treatment duration with rivaroxaban was 544 days (25th and 75th percentile 444/639d) for all patients. During follow-up, the overall persistence with rivaroxaban therapy was 81.5% (223/1204 patients discontinued rivaroxaban) and similar for patients switched from VKA to rivaroxaban or newly treated rivaroxaban patients (82.0% vs 81.1%).
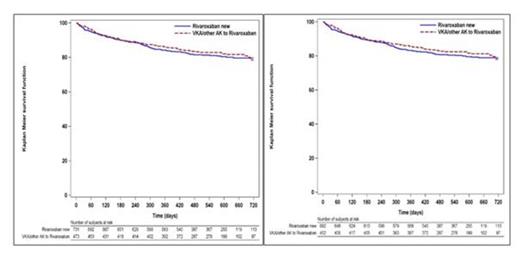
In the intention-to-treat analysis, rates of treatment discontinuation per 100 patient-years were assessed as a Kaplan–Meier time-to-first-event analysis and found to be 13.6 [95% CI 11.8–15.4] for all patients and similar for newly treated rivaroxaban patients (14.1 [95% CI 11.9–16.7]) and patients switched from VKA to rivaroxaban (12.7 [95% CI 10.1–15.7]; p = 0.35; Figure 1a). This finding did not change if only patients with a completed 12-month follow-up were assessed (Figure 1b).
Discontinuation rates were highest in the first 6 months of treatment (9.9% [95% CI 7.7–12.1%] for patients newly treated with rivaroxaban and 10% [95% CI 7.3–12.7%] for patients switched from VKA pretreatment to rivaroxaban) and declined similarly in both subgroups over time (for 6–12 months: 6% [95% CI 5.5–6.4%] and 3.9% [95% CI 3.5–4.4%], respectively; after 12 months: 4.4% [95% CI 3.9–5%) and 7.7% [95% CI 6.0–9.2%], respectively).
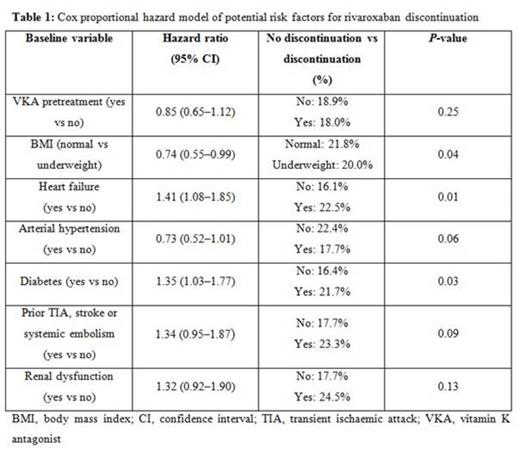
Most common reasons for treatment discontinuations were bleeding complications (30% of all discontinuations), followed by other side-effects (24.2%) and diagnosis of stable sinus rhythm (9.9%). Within the group of 67 bleeding complications, according to the International Society on Thrombosis and Haemostasis bleeding definition, 14 were major and 53 were non-major clinically relevant bleeding events that led to treatment discontinuation. A history of chronic heart failure (HR 1.43; 95% CI 1.09-1.87; p=0.009) or diabetes (HR 1.39; 95% CI 1.06-1.82; p=0.018) were the only statistically significant baseline risk factors for rivaroxaban discontinuation. After discontinuation of rivaroxaban, patients received antiplatelet therapy (31.8%), VKA (24.2%), another NOAC (18.4%), heparin (9.9%) or nothing (15.7%).
Conclusion: Our data indicate that overall persistence with rivaroxaban therapy is high, with a discontinuation rate of approximately 15% in the first year of treatment and few additional discontinuations thereafter. Non-major bleeding is the most common reason for riaroxaban discontinuation and nearly 50% of all discontinuing patients receive only antiplatelet or no antithrombotic treatment.
Kaplan–Meier analysis of persistence to rivaroxaban treatment for all patients (left diagram) and for all patients who were observed for at least 12 months (right diagram), according to VKA pretreatment
Kaplan–Meier analysis of persistence to rivaroxaban treatment for all patients (left diagram) and for all patients who were observed for at least 12 months (right diagram), according to VKA pretreatment
Beyer-Westendorf:Bayer: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Boehringer Ingelheim: Honoraria, Research Funding. Weiss:Boehringer Ingelheim: Honoraria; Bayer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal