Abstract
Background: It is well known that atrial fibrillation (AF) induces a hypercoagulable state, which significantly increases stroke risk in patients with AF contributing to morbidity and mortality in these patients. Active coagulation factors can also provoke diverse cellular responses through stimulation of protease-activated receptors (PARs). In the heart and vessels, coagulation factor mediated PAR activation may provoke and mediate pro-inflammatory and tissue remodeling responses, potentially contributing to organ damage. We hypothesized that the onset and progression of AF, may be affected by hypercoagulability-mediated cell signaling responses, in the heart.
Methods and results: To study the potential role of PARs in the structural remodeling process that renders the atria more prone to AF we first investigated whether thrombin or factor Xa could induce atrial fibroblast remodeling. In isolated rat cardiac fibroblasts, thrombin enhanced the phosphorylation of the pro-fibrotic signaling molecules Akt and Erk, and increased expression of TGFβ1 (2.7 fold) and the pro-inflammatory factor monocyte chemo-attractant protein-1 (6.1 fold). Thrombin also increased the incorporation of 3H-proline suggesting enhanced collagen synthesis by cardiac fibroblasts (2.5 fold). Differentiation towards myofibroblasts was indicated by increased expression of smooth muscle actin (2 fold). All effects could be prevented by the direct thrombin inhibitor dabigatran and comparable results were obtained for stimulation with factor Xa and inhibition with rivaroxaban, respectively.
Next we studied whether enhanced stimulation of PARs by chronic elevation of thrombin levels would lead to an enhanced vulnerability to AF in transgenic mice. In mice with enhanced thrombin activity due to a mutation in the thrombomodulin gene resulting in impaired thrombin inhibition (TMpro/pro), inducibility of AF episodes provoked by burst pacing was higher (6 out of 10 versus 1 out of 10 in wild type) and the duration of AF episodes was longer (episodes >2s in 6 out of 10 versus 0 out of 10 in wild type). Finally, we showed that inhibition of the coagulation cascade attenuated the development of AF in a goat model of AF. In 6 goats with persistent AF and treated with the anticoagulant nadroparine (4 weeks, 150 IU/kg twice daily) the complexity of the AF substrate was less pronounced compared to control animals. The conduction heterogeneity and block were 33% shorter in the nadroparine treated animals (maximal conduction time 23.3±3.1ms in control versus 15.7±2.1ms in nadroparine, p<0.05) and AF-induced a-SMA expression and endomysial fibrosis were less pronounced.
Conclusion: The hypercoagulable state during AF provokes pro-fibrotic and pro-inflammatory responses in cardiac fibroblasts, as well as promotes the development of a substrate for AF in transgenic mice and in goats with persistent AF. Together, these results strongly support the role of hypercoagulability and PAR activation in the development of a substrate for AF. In addition, direct anticoagulant treatment may protect against AF-related cellular atrial remodelling.
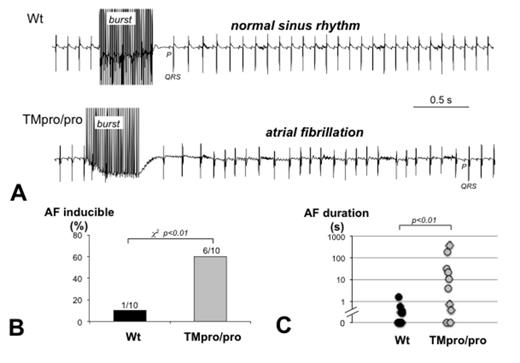
Enhanced AF inducibility and prolonged AF duration in TMpro/pro mice. Transesophageal stimulation was used to test AF inducibility. A surface electrocardiogram (lead I, sampled at 2.5 kHz) was recorded to detect AF. A) Traces show an example of a Wt mouse, returning to normal sinus rhythm immediately after the burst (upper panel) and a TMpro/pro mouse, showing a 3s episode of AF before returning to sinus rhythm (lower panel). In both cases, the first P wave observed after the burst is indicated. B) AF was inducible in 1 out of 10 Wt mice and 6 out of 10 TMpro/pro mice. C) Distribution of the longest AF episode duration observed in each Wt and TMpro/pro mouse.
Enhanced AF inducibility and prolonged AF duration in TMpro/pro mice. Transesophageal stimulation was used to test AF inducibility. A surface electrocardiogram (lead I, sampled at 2.5 kHz) was recorded to detect AF. A) Traces show an example of a Wt mouse, returning to normal sinus rhythm immediately after the burst (upper panel) and a TMpro/pro mouse, showing a 3s episode of AF before returning to sinus rhythm (lower panel). In both cases, the first P wave observed after the burst is indicated. B) AF was inducible in 1 out of 10 Wt mice and 6 out of 10 TMpro/pro mice. C) Distribution of the longest AF episode duration observed in each Wt and TMpro/pro mouse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal