Abstract
Introduction:Atypical hemolytic uremic syndrome (aHUS) is a rare, genetic, life-threatening disease of chronic, uncontrolled complement activation leading to thrombotic microangiopathy, renal, and other end-organ damage. Started in April 2012, the aHUS Registry is an observational, non-interventional, multicenter, global registry designed to collect information on patients (pts) with aHUS. By making follow-up data on the aHUS indication for eculizumab (ECU) available, the Registry fulfills postmarketing regulatory requirements while also highlighting the need for and benefit of a sponsor/academia partnership. An independent Scientific Advisory Board ensures data are made accessible for publication. Herein, we report baseline demographics and clinical characteristics of pts enrolled in the aHUS Registry.
Methods:Pts of all ages with a clinical diagnosis of aHUS (irrespective of treatment) are eligible for enrollment into the Registry. An identified complement abnormality is not required. Demographic, medical and disease history, treatment, and efficacy and safety outcomes data are collected at Registry enrollment and prospectively thereafter.
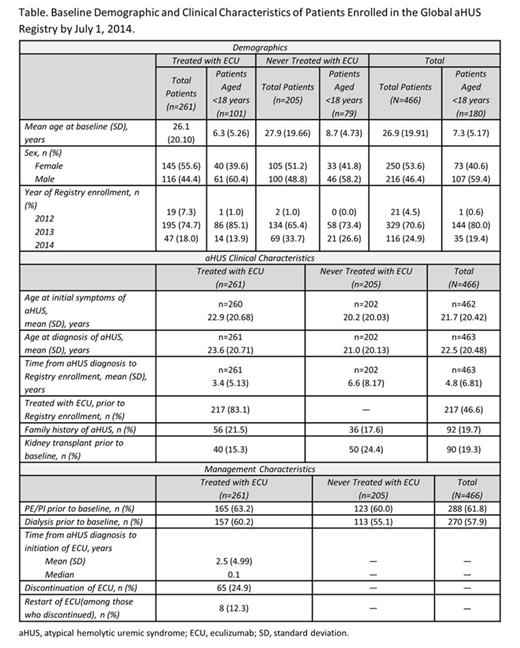
Results:By July 1, 2014, 466 pts (from Australia, Austria, Belgium, Canada, France, Germany, Israel, Italy, Russia, Spain, Sweden, Switzerland, the UK, and USA) were enrolled in the aHUS Registry. Of these, 261 (56.0%) were treated with ECU and 286 (61.4%) were ≥18 years of age. Family history of aHUS, prior kidney grafts, dialysis, plasma exchange/plasma infusion (PE/PI), and renal impairment were assessed at baseline in ECU- and non–ECU-treated pts (Table). Mean time from aHUS diagnosis to ECU initiation was 2.5 years. Sixty-five pts (24.9%) discontinued ECU; of these, 8 (12.3%) restarted.
Conclusions:Since the public disclosure of data from the last aHUS Registry update, pt enrollment has continued to increase. Ongoing and future analyses will further clinicians’ understanding of the history and progression of aHUS. Additional clinical sites are encouraged to enroll pts into the aHUS Registry to facilitate knowledge acquisition and optimization of pt care and quality of life.
Licht:Achillon Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Ardissino:Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ariceta:Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cohen:Alexion Pharmaceuticals: Consultancy. Gasteyger:Alexion Pharma International Sàrl: Employment, Equity Ownership. Greenbaum:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ogawa:Alexion Pharmaceuticals: Employment. Schaefer:Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Vande Walle:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Fremeaux-Bacchi:Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CLS Behring: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal