Abstract
Background: Cancer patients who are receiving chemotherapy are at risk for influenza infections and its severe complications. Although influenza vaccination is recommended, preliminary data have raised questions about the immune response to vaccination in such patients. Furthermore, recent studies suggest that some tyrosine kinase inhibitors (TKIs) induce significant impairment of B cell responses and reduce vaccine efficacy. The two-dose influenza vaccination is proposed to be one of the strategies to increase the efficacy of influenza vaccination in cancer patients, but its efficacy has yet to be confirmed.
Methods: This was a prospective multicenter study to evaluate the efficacy of the two-dose influenza vaccination in cancer patients receiving chemotherapy. We administered a triple-strain (A/California/7/2009 [H1N1], A/Texas/50/2012 [H3N2], and B/Massachusetts/2/2012) 2013/14 influenza vaccine to patients with pathologically confirmed malignancies. All patients were under the treatment with chemotherapy including cytotoxic agents, rituximab, or TKIs. Vaccination was performed on days when chemotherapy was not given (except for TKIs). The second vaccination was performed in patients who did not respond to all three strains after the first vaccination. Hemagglutination inhibition (HI) titers were measured, and 1:40 or greater HI titers were considered protective because these titers were reported to be associated with at least a 50% reduction in influenza infection in general populations. HI titers were measured within two weeks prior to the first vaccination, within 3-5 weeks after each vaccination, and at the end of the influenza season in May 2014.
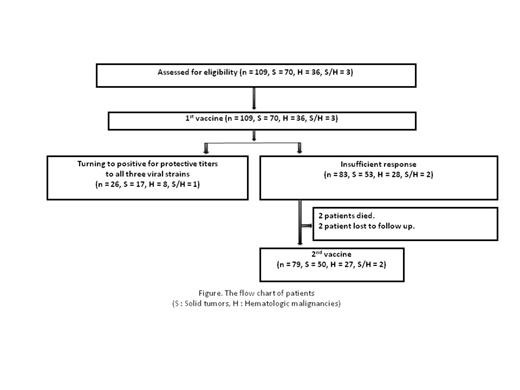
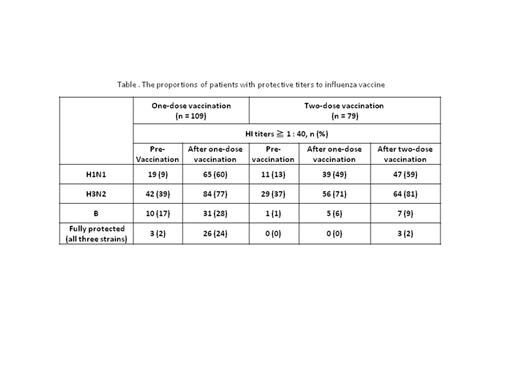
Results: A total of 109 patients (median age 61 years [range 21–89 years]) were enrolled; 55 females and 54 males, 36 hematological malignancies (multiple myeloma [n=15], malignant lymphoma [n=10], chronic myeloid leukemia [n=10], and acute lymphoblastic leukemia [n=1]), 70 solid tumors (breast [n=14], colorectal [n=11], biliary tract [n=10], and others [n=35]), and both cancer types (n=3). The flow chart of patients is shown in Figure. Table lists the proportions of patients with protective titers. The proportion of patients who had protective titers against all three strains was increased from 2 to 24% by the first vaccination. Similarly patients with protective titers against individual strains were increased; further 3-10% of patients achieved protective titers against individual strains after the second vaccination. When we compared the proportion of patients with protective titers against all strains after the first vaccination between the two disease types, the efficacy was similar; 22% (8/36) in hematological malignancies and 24% (17/70) in solid tumors (p=1.0). Similarly, there were no significant differences after the second vaccination between the two disease types. We also assessed differences in the response between patients treated with TKIs alone and those treated with other therapies. After the first vaccination, the proportion of patients who had protective titers against all three strains was 32% (6/19) for TKIs alone while it was 22% (20/90) for other therapies (p=0.39). Also there were no differences after the second vaccination regardless of the use of TKIs. When the effect on immunization of age, leukocyte and lymphocyte counts, and duration from chemotherapy to the first vaccination was evaluated, none of these factors influenced vaccine response. Serious adverse events associated with influenza vaccination were not reported, even among patients who received the two-dose vaccination.
Conclusions: We recommend influenza vaccination for cancer patients who are receiving chemotherapy, and two-dose vaccination might be an effective strategy to augment vaccine efficacy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal