Abstract
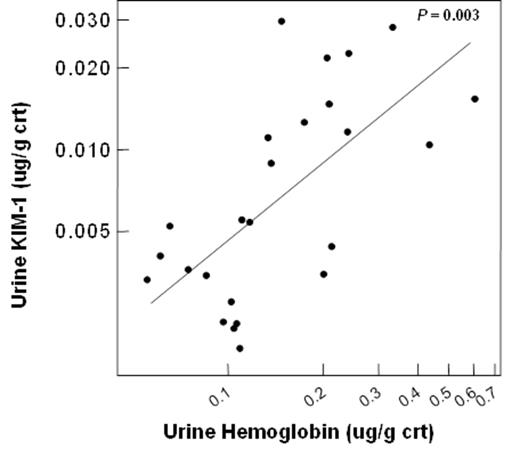
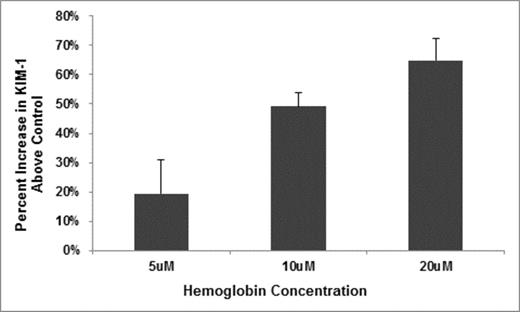
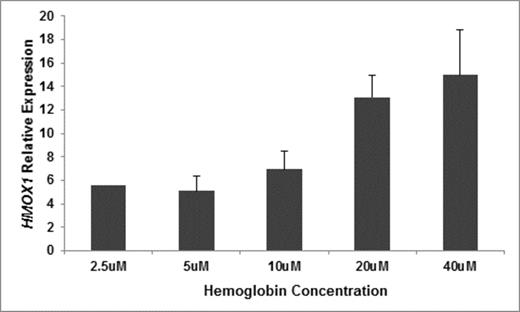
We recently reported that hemoglobinuria is associated with chronic kidney disease (CKD) stage and progression in sickle cell disease (SCD) (Saraf et al. BJH 2014). To further investigate a potential role of cell-free hemoglobin in SCD nephropathy, we measured urinary concentrations of kidney injury molecule-1 (KIM-1), a biomarker of tubular injury, and nephrin, a marker of glomerular injury, in 32 University of Illinois at Chicago (UIC) patients. Urine KIM-1 concentration directly correlated with increasing urine cell-free hemoglobin concentration (P = 0.003) (Figure 1) but urine nephrin concentration did not. To determine biological responses of tubular cells to cell-free hemoglobin, we added lyophilized hemoglobin to cultured human kidney-2 (HK2) tubular cells. Supernatant KIM-1 concentrations increased progressively with increasing cell-free hemoglobin exposure (Figure 2) while total cell number and cell viability were stable. Using a fluorescein-labeled hemoglobin assay, we found that cell-free hemoglobin bound to HK2 cells in a competitive manner. To determine whether enzymes for metabolizing hemoglobin and protecting from reactive oxygen species are affected by exposure of HK2 cells to cell-free hemoglobin, we evaluated candidate gene expression using rt-PCR. The relative expression of heme oxygenase-1 (HMOX1) (Figure 3) progressively increased with increasing cell-free hemoglobin dose while expression of superoxide dismutase 1 and 2, catalase, glutathione reductase, and glutathione synthetase did not change.
We then focused on variants of HMOX1 in a cohort of 247 UIC SCD patients. Genotyping was carried out using Affymetrix Axiom genome-wide Pan-African array in DNA isolated from peripheral blood mononuclear cells. Examination of 11 tag SNPs within +/-10 kb of HMOX1 identified a SNP in the promoter region of HMOX1 (rs743811, minor allele frequency 0.14) that had a significant association with CKD stage (β = -1.0, P = 0.00032) and non-significant associations of the same direction with end stage renal disease (ESRD) (β = -0.9, P = 0.2) and hemoglobinuria (β = -0.2, P = 0.6). Validation studies were conducted in 517 SCD patients from the Walk-Treatment of Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy (Walk-PHaSST) cohort. Genotyping was performed using the Illumina Human 610-Quad SNP array and imputed using the HapMap II reference panels for HMOX1 tag SNPs. HMOX1 rs743811 had a significant associated with ESRD (β = -1.6, P = 0.014) and non-significant associations of a similar direction with CKD stage (β = -0.2, P = 0.3) and hemoglobinuria (β = -0.3, P = 0.3).
Homozygosity or compound heterozygosity for the G1 and G2 variants of APOL1, encoding apolipoprotein 1, have been implicated in CKD in African Americans with and without SCD (Ashley-Koch et al. BJH 2011), and we therefore examined their association with hemoglobinuria-associated CKD in the UIC cohort. The S342G and I384M substitutions are in almost complete linkage disequilibrium and are termed G1; the deletion of two amino acids, N388 and Y389, is termed G2. The G2 variant was further imputed using reference panels of the 1000 Genomes Project. Homozygosity or compound heterozygosity of the G1/G2 variant was associated with hemoglobinuria (β = 2.0, P = 0.00018), CKD stage (β = 1.0, P = 0.022), and ESRD (β = 1.9, P = 0.036). For validation studies in Walk-PHaSST, the G1 and G2 variants of APOL1 were imputed using the 1000 Genomes Project. Homozygosity or compound heterozygosity of G1/G2 in the Walk-PHaSST cohort was associated with hemoglobinuria (β = 0.7, P = 0.021), but associations of a similar direction with CKD stage (β = 0.2, P = 0.6) and ESRD (β = 1.1, P = 0.3) were not statistically significant.
Our findings are consistent with the possibility that cell-free hemoglobin contributes to sickle cell nephropathy through tubular injury. A SNP in the promoter region of HMOX1 is associated with CKD stage in the UIC cohort and ESRD in the Walk-PHaSST cohort, raising the possibility that altered HMOX1 expression can have a role in SCD-associated CKD. Our results also point to a novel association of the G1/G2 variants of APOL1 with cell-free hemoglobin-mediated CKD in SCD subjects. Future studies to explore the potential roles of HMOX1 and APOL1 in cell-free hemoglobin-associated sickle cell nephropathy are warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal