Abstract
Background
BCR-ABL1 kinase domain (KD) mutations are the most common known cause of resistance to tyrosine kinase inhibitors (TKIs) in CML. Mutation analysis is critical for selection of subsequent TKI therapy after treatment failure. Low level and compound mutants (>1 KD mutation in the same molecule) may also lead to therapy failure. However, compound and multiple polyclonal mutants cannot be distinguished by conventional methods as they determine the average genotype of all molecules. Next generation sequencing (NGS) has the potential to sensitively detect these mutants, however sequencing and PCR errors confound the detection of true, low level mutants using current approaches. Indeed, we demonstrated that the reported frequency of BCR-ABL1 compound mutants may be over estimated due to PCR recombination artifacts that mimic compound mutations (Parker Blood 2014). More reliable methods are needed to appropriately assess the impact of various mutations on patient (pt) outcome.
Aim
To develop a clinically applicable NGS assay that can robustly distinguish BCR-ABL1 compound and polyclonal mutants.
Method
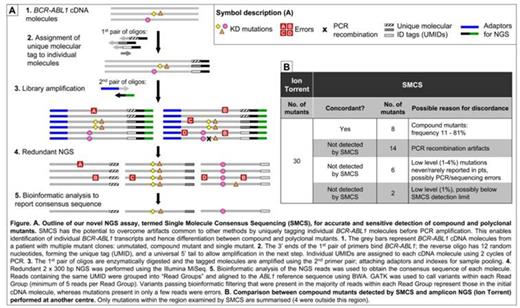
We have developed a novel NGS assay termed Single Molecule Consensus Sequencing (SMCS) that involves tagging individual BCR-ABL1 cDNA molecules before library amplification, enabling identification and elimination of most PCR and sequencing errors. NGS was performed on the Illumina MiSeq, 2 x 300 bp; aa 244 - 407 of the KD was examined. Reads derived from an initial BCR-ABL1 molecule are identified bioinformatically by virtue of sharing the same tag sequence. The consensus sequence of reads with the same tag is determined using automated variant calling and filtering algorithms. The consensus sequence represents the sequence of the initial BCR-ABL1 cDNA molecule (Fig A).
Results
To test the validity of SMCS, we examined 10 samples lacking KD mutations and 5 mock samples created by mixing compound mutant plasmids or pt samples. Examination of raw sequencing reads revealed a complex spectrum of mutants, similar to previous clinical reports. SMCS enabled bioinformatic filtering of these artifacts, largely eliminating PCR and sequencing error, and exclusively reported the compound and polyclonal mutants known to be present in the mock samples. We estimated the background error rate to be ~2x10-5 per base. The error spectrum was consistent with DNA damage causing first round PCR errors.
SMCS was used to retrospectively examine samples of 46 pts (36 CP, 2 AP, 8 BP) who were resistant to ≤4 TKIs (1st and 2nd generation). 71 mutations were previously detected by Sanger sequencing in these samples, collected before starting next line TKI. Within the region examined using SMCS, there was 100% detection concordance with Sanger sequencing.
We compared the results of SMCS with an amplicon NGS method performed at another centre for 24/46 pts (Ion Torrent, depth ~10000). Ion Torrent detected 34 compound mutants in 24 pts. Of the 30/34 that were within the region examined by SMCS we only detected 8. Based on observations in Parker Blood 2014, 14 of the 22 compound mutants not detected by SMCS were likely to be PCR recombination artifacts. The other 8/22 were low level (1 - 4%) and most (6/8) involved mutations rarely/never reported in TKI resistant pts so may also be artifacts (Fig B). We detected 3 additional compound mutants in these 24 pts, plus 5 in the remaining 22/46 pts. The compound mutants detected by SMCS were consistent with the pts' TKI treatment history.
Conclusion
We demonstrated detection of BCR-ABL1 compound and polyclonal mutants in pt samples using a novel NGS assay that has the potential to overcome technical artifacts generated with other published methods. Whilst there is no gold standard method that can accurately detect low level compound mutations, SMCS has correctly identified sequencing and PCR recombination artifacts using mock samples. The accuracy and clinical utility of SMCS for sensitive compound and polyclonal mutant detection is currently being validated in another group of 200 imatinib resistant pts. The frequency of compound mutants detected in pts with >1 mutation by SMCS in the current analysis (35%) is approximately half of that reported previously, which suggests the published frequency may have been overestimated. Our novel assay takes an important step towards enabling a more concrete understanding of the mutation spectra in pts and their association with resistance.
Yeung:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Lustgarten:ARIAD Pharmaceuticals Inc: Employment, Equity Ownership. Hodgson:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Rivera:ARIAD Pharmaceuticals Inc: Employment, Equity Ownership. Hughes:Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Ariad: Honoraria, Research Funding. Branford:Novartis: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Ariad: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal