Abstract
Background. Patients who experienced leukemia relapse after allogeneic hematopoietic stem cell transplantation (HSCT) have a dismal prognosis. Strategies to prevent or overcome post-transplant disease recurrence are limited and their efficacy still remains unclear. Recently, HSCT using haploidentical T-replete source has found increasing feasibility and acceptance thanks to new transplant platforms (Luznik L. et al., Biol Blood Marrow Transplant, 2008; Peccatori J. et al., Leukemia, 2014), allowing lower rates of graft-versus-host disease (GvHD) and non-relapse mortality (NRM). We evaluated the outcome of haploidentical transplant approach performed as second transplant (HSCT2) for patients who suffered of acute leukemia (AL) relapse after a first allogeneic HSCT (HSCT1).
Methods. We analyzed 42 consecutive patients (median age 48 years) who underwent HSCT2 from a haploidentical donor, different from the previous one, between 2007 and 2013 in our Institute. Patients suffered of acute myeloid leukemia (n=31), acute lymphoblastic leukemia (n=9) or biphenotypic AL (n=2) relapsing after HSCT1 (matched related: 21; unrelated: 17; mismatched related: 4; umbilical cord blood: 4). Thirty-seven patients (88%) received re-induction therapy before HSCT2: 13 of them achieved complete remission (CR), 16 did not respond and 8 patients were transplanted directly in aplasia. In 5 cases HSCT2 was performed without re-induction therapy, in active disease (AD). Median Hematopoietic Cell Transplant-Co-morbidity Index was 2 (range 0-6) at time of HSCT2.
All patients received treosulfan (14 g/m2 for 3 days) and fludarabine (30 mg/m2 for 5 days) as part of preparative regimen for HSCT2; conditioning was intensified with total body irradiation (4 Gray) in 18 (43%) or with melphalan (70 mg/m2 for 2 days) in 6 patients (14%). GvHD prophylaxis consisted of mycophenolate mofetile and sirolimus combined with pre-transplant anty-thymocyte globuline (n=36) or with post-transplant cyclophosphamide (n=6). Unmanipulated peripheral blood stem cells were used as graft source in all cases.
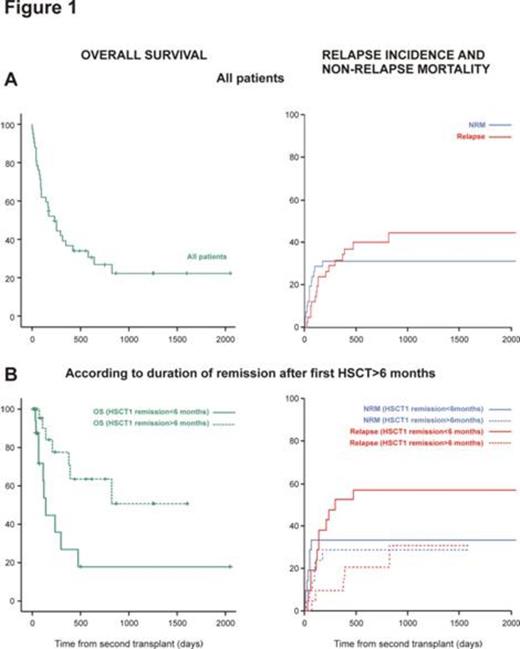
Results. Three out of 42 patients (7%) experienced early death in aplasia, while all the others achieved neutrophil engraftment (n=39, 93%), with no primary graft rejection. Complete remission of disease at day +30 was reached in 37 out of 39 evaluated cases (95%). Estimated overall survival (OS) and leukemia-free survival (LFS) (± SE) rates at 1 year from HSCT2 were 37% (± 8%) and 35% (± 7%), respectively (Figure 1A). One-year relapse incidence (± SE) amounted to 32% (± 7%) and NRM (± SE) to 31% (± 7%) (Figure 1A) (7 patients died for infectious, 5 for GvHD and 1 for central nervous system toxicity). Sixteen patients (38%) experienced of acute GvHD grade II-IV and 20 (47%) of chronic GvHD. Disease status at HSCT2 (AD vs CR) had no impact on OS (HR:1.26, 95% CI 0.58-2.76, p=0.564) and relapse (HR:1.35, CI 95% 0.46-3.76, p=0.61). Conversely, time of remission from HSCT1>6 months provided the better outcome in terms of estimated OS (±SE) at 1 year (50% ± 10%) compared to patients with shorter duration of remission (18% ± 9%) (HR:0.49, 95% CI 0.24-1.02, p=0.05) and reduced risk of relapse (HR:0.28, 95% CI 0.11-0.75, p=0.011) (Figure 1B). After a median follow-up time of 22 months (range 6-68), 11 patients (26%) were alive with sustained leukemia remission.
Conclusions. These results demonstrate the feasibility of HSCT2 from a haploidentical donor as treatment for AL relapse after initial allogeneic transplant, showing rates of OS, LFS and NRM comparable to the reported in literature for HSCT2 from matched related or unrelated graft, using the same or different donor (Christopeit M. et al., J Clin Oncol, 2013). Interestingly, disease status at HSCT2 did not influence the outcome of transplant. While, in accordance with previous reports, duration of remission after HSCT1 is the major factor involved in predicting outcome of a second transplant due to an increased relapse incidence after HSCT2 in patients with shorter HSCT1 remission, reflecting diseases biologically different. If confirmed in a larger cohort, these data could be useful in decision making after HSCT1 failure for disease recurrence.
Bonini:MolMed S.p.A.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal